Clinical, neurological, and neurophysiological evaluation of the efficiency of motor rehabilitation in children with cerebral palsy using robotic mechanotherapy and transcutaneous electrical stimulation of the spinal cord
- Authors: Ikoeva G.A.1,2, Nikityuk I.E.2, Kivoenko O.I.2, Moshonkina T.R.3, Solopova I.A.4, Sukhotina I.A.3, Vissarionov S.V.2,1, Umnov V.V.2, Gerasimenko Y.P.3
-
Affiliations:
- North-Western State Medical University n.a. I.I. Mechnikov
- The Turner Scientific and Research Institute for Children’s Orthopedics
- Pavlov Institute of Physiology of the Russian Academy of Sciences
- Institute for Information Transmission Problems of the Russian Academy of Sciences
- Issue: Vol 4, No 4 (2016)
- Pages: 47-55
- Section: Articles
- URL: https://journals.eco-vector.com/turner/article/view/5895
- DOI: https://doi.org/10.17816/PTORS4447-55
- ID: 5895
Cite item
Abstract
Introduction. Rehabilitation of patients with cerebral palsy (CP) remains a very difficult task. Stable and growing movement restrictions in such patients cause a life-long need for treatment and rehabilitation. Neurorehabilitation of children with CP at various stages includes not only traditional physical rehabilitation methods, but also extensive use of robotic mechanotherapy techniques and new technologies in the field of neurophysiology. One of such technology is non-invasive percutaneous electrical stimulation of the spinal cord.
Aim of the study: To assess the effect of transcutaneous electrical stimulation of the spinal cord to improve the motor function of children with spastic diplegia using the “Lokomat” robotic mechanotherapy system.
Materials and methods. A clinical rehabilitation study of 26 patients aged 6–12 years with CP was conducted. The treatment group included 11 patients who received one course of robotic mechanotherapy using the “Lokomat” system combined with transcutaneous electrical stimulation of the spinal cord. The control group included 15 patients who received one course of robotic mechanotherapy only.
Results. A comparative analysis of the two groups based on the results of clinical examinations using specific scales (GMFCS, GMFM-88, Modified Ashworth Scale of Muscle Spasticity), locomotor tests (L-FORCE, L-ROM), and evaluations of muscle activity using electromyography showed that one course of rehabilitation resulted in improvement in motor function in all patients of both groups, but positive dynamics were more significant in the treatment group that underwent percutaneous electrical stimulation of the spinal cord.
Conclusion. Based on clinical data, changes in indicators of the locomotor tests L-FORCE and L-ROM, as well as assessment of changes in muscle activity, showed that motor rehabilitation of children with spastic diplegia using the “Lokomat” robotic mechanotherapy system combined with transcutaneous electrical spinal cord stimulation was more effective than robotic mechanotherapy only.
Full Text
Introduction
Infantile cerebral paralysis (ICP) is a group of syndromes of the central nervous system lesions associated with non-progressive disease conditions caused by exposure of the developing brain of a fetus or child during the prenatal, intranatal, or early postnatal periods, to damaging agents [1]. Brain damage manifests as a disorder of muscle tone, formation of paresis and paralysis of limbs, and body position and movement disorders, leading to limitations of the patient’s social activities [1]. Movement disorders associated with ICP are usually accompanied by cognitive and behavioral impairment, speech disorders, disorders of hearing and visual functions, and epileptic seizures [1]. Persistent movement disorders lead to the formation of multiple contractures and secondary deformities of bone segments of the limbs, which are formed and progress during the course of growth and development of the child [2]. Although ICP is not a progressive disease, its complications can aggravate during the course of the patient’s disability. Persistent and growing movement restrictions in patients with cerebral paralysis result in the requirement for lifelong treatment and rehabilitation.
The rehabilitation treatment of ICP patients remains a very challenging task. The therapeutic methods administered to ICP pediatric patients are usually aimed to control spasticity, hyperkinesis, and epileptic seizures, but do not yield the desired results and success with regard to the management of orthopedic complications. Therefore, physical rehabilitation in these patients is very challenging and does not always result in the improvement of motor function [3-6]. Recent studies have shown that the use of contemporary high-tech and associated forms of assistance can bring tangible results in solving this problem. A treatment strategy, which includes neurological, orthopedic, and neurosurgical treatment, followed by neurorehabilitation has been recognized as the most effective treatment option for these patients to date [6-8].
The neurorehabilitation of pediatric patients with cerebral paralysis not only includes the traditional means of physical rehabilitation, but also makes extensive use of robotic technologies [9-12] and other novel advances in the field of neurophysiology. One such technology is the non-invasive electrical stimulation of the spinal cord [13]. Studies focusing on percutaneous spinal electrical stimulation in ICP patients, which indicate the efficiency and prospects of this technique in the motor rehabilitation of pediatric patients with spastic paralysis, have emerged in the literature [14].
The study aimed to examine the effect of transcutaneous electrical stimulation of the spinal cord (TESSC) during robotic mechanotherapy using the “Lokomat” system on motor functions of pediatric patients with spastic diplegia.
Materials and methods
In total, 26 patients aged 6–12 years (mean age ± standard deviation, 8.6 ± 3.4 years) with spastic diplegia, a type of ICP, were enrolled in the present study. The degree of mental development disorder was assessed as mild or moderate, and all patients were able to establish contact and could perform the instructor’s tasks well. All patients previously received the step-by-step surgical or conservative orthopedic treatment aimed at eliminating muscle spasticity and contractures of the lower extremities. After each step, patients underwent rehabilitation to enhance motor activity. Depending on the variants of restorative treatment, patients were divided into two groups: a study group and a control group. The study group included 11 pediatric patients who received 15 procedures with robotic mechanotherapy using the “Lokomat” system, each lasting 45 minutes, in combination with TESSC. The control group included 15 pediatric patients who also received 15 procedures of the robotic mechanotherapy, each lasting 45 minutes, but without the use of TESSC [15].
To evaluate the treatment outcomes, the following methods were used:
- The Modified Ashworth Scale of Muscle Spasticity. The degree of muscle spasticity of the lower limbs was evaluated before robotic mechanotherapy and after its completion [16].
- The Gross Motor Function Classification System (GMFCS) for the assessment of the level of motor skill development and the Gross Motor Function Measure (GMFM). The evaluation of the motor function level and changes in gross motor functions in pediatric patients with cerebral paralysis were completed at the beginning and at the end of the mechanotherapy course [17].
- The L-FORCE test is a standard test included in the software of the simulator “Lokomat”. It evaluates isometric muscle strength in newton meters (Nm) in four groups of muscles: femoral flexors/extensors and tibial flexors/extensors on the right and left lower extremities.
- The L-ROM test is a standard test included in the software of the “Lokomat” system. It estimates the amount of passive motion in the flexion and extension of the knee and hip joints (in degrees).
- Assessment of lower limb muscle activity.
Assessment of muscle activity was performed via telemetry recording of the electrogenesis of lower limb muscles during the patient’s independent step movements on the “Lokomat” system treadmill belt. The patient was secured in a simulator “Lokomat” and raised above the treadmill so that he/she could make step movements on the moving belt. The treadmill belt speed was selected so that the patient did not lose contact with the belt while walking, and it ranged from 0.3 to 1.5 km/h [18].
Assessment of muscle activity was performed as follows: signal filtering from the power-supply noise of 50 Hz was performed using the following formula:
Af(t) = A(t) – A(t – 0.02),
where A(t) is the initial EMG signal.
Thus, from the current signal, the same signal shifted forward by 0.02 was subtracted. Also, the signal was filtered from the low-frequency artifacts to 20 Hz. The time interval in which the signal was studied was approximately 20 s and was selected manually to ensure a well-established periodic motion during this interval. The spectrum of the EMG signal had a limited range of 20–500 Hz. Therefore, according to the Nyquist-Shannon sampling theorem, the frequency of signal digitizing was chosen as 1 kHz. The Fourier spectrum was created within the range of 20–500 Hz.
The Fourier transform of the EMG signal A(t)
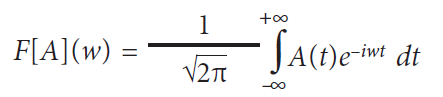
forms the basis of the wavelet decomposition:

A special case of the wavelet decomposition is a short-time Fourier transform

where ψ(τ – t) is the window function, in this case, 500 points (0.5 s). The Fourier transform is constructed for this window with a further bias of 10 points (0.01 s). The window is not of rectangular type, but with edges smoothed to zero with the help of

where t1 is the beginning of the active window, t2 is the end of the active window, n is logarithmic decay rate of the signal emitted, and A(t) is the initial EMG signal.
Good results in the construction of the picture of wavelet decomposition give the following parameter values: t1 = 0.225 c (225th point of the window, the zero time is taken each time the beginning of the window), t2 = 0.275 c (275th point of the window), n = 7. Using these parameters, the quality correspondence of the short-time Fourier transform to the wavelet decomposition, which is constructed by standard methods, such as the Morlet wavelet, was achieved. Thus, 500 points, of which 50 are active, were allocated for the window, and outside of the active site there is a sharp decrease in the signal to avoid the oscillatory artifacts at the low frequency region of the Fourier spectrum. The Fourier transform was constructed in the window described above, and the dependences of the coefficients of the Fourier spectrum were calculated based on time and frequency. Spectral coefficients were displayed on the time-frequency plane where the point color corresponds to the coefficient value, and thus the characteristic pattern of the wavelet decomposition was obtained [19] (Fig. 1)
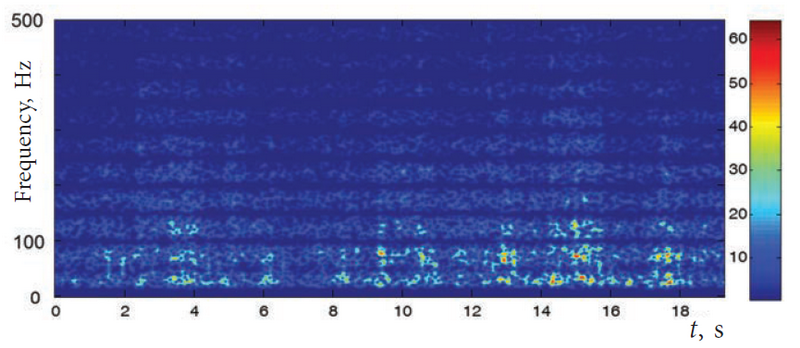
Fig. 1. Characteristic pattern of the wavelet decomposition of the EMG signal. The record of VL of a healthy child’s right leg is presented. On the right, the scale of correspondence of the spectral coefficient magnitude and the scale level of the gray shades of the corresponding points are shown
EMG signal level was normalized so that the maximum coefficient of the Fourier spectrum of the entire record was equal to 64, which corresponds to the white color of the gray shade scale.
The relative time of the active state
Active state of the muscle and the relative time of the active state were determined on the wavelet decomposition. For this, the threshold that distinguishes between the resting and active states was determined. The average power of the spectrum was determined as

where the summation is in the area of time t and I is the total spectral power versus time.
In addition, the average power spectrum, Icp(200÷400), in the range of 200–400 Hz on the selected time interval was calculated. A high frequency range was chosen owing to the high frequency signal components of the active force EMG. In addition, the EMG spectrum in the high-frequency range correlates well with the power developed by the muscle.
The threshold for determining the active state of muscle was analyzed as the following ratio:
L = (1 + 0.2 (5.5 – Icp)/4.5) Icp(200÷400).
The coefficients were chosen such that the threshold was reduced to 0.8Icp(200÷400) with a large Icp, while it was increased to 1.2Icp(200÷400) with a small Icp.
At each moment, for the area under study of the wavelet decomposition, the threshold L and the spectral power were compared at a given time in the range of 200–400 Hz. When this power value was above the threshold, the time point was considered “active”, and when this power value was below the threshold, the time point was considered “passive” (Fig. 2.).
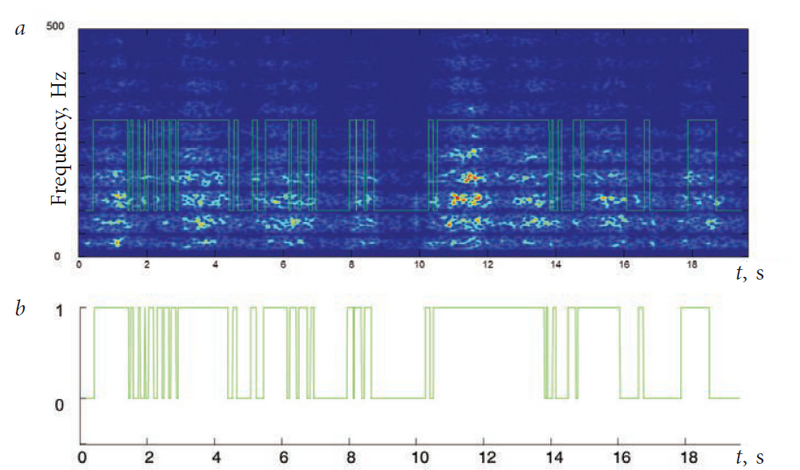
Fig. 2. Example of release of the active and the passive state: a) extracting the state of the muscle from the EMG wavelet decomposition; b) the extracted dependence of the state of muscle activity (0 - passive, 1 - active)
Relative duration of activity was defined as the ratio of active time to the duration of the entire study area of the electromyogram as follows: τ = ta/t.
All studies were performed twice in each patient, once at the beginning and once at the end of robotic mechanotherapy. For comparison, standard values of the studied parameters were determined in 10 healthy children of the same age group.
Results and discussion
In the present study, pediatric patients with motor skill development levels of 2–4 on the GMFCS scale were included. The motor skill development levels remained constant throughout the study, as they cannot change significantly in a short period of time, even with very intensive rehabilitation. Results of the motor function evaluations on the GMFCS scale in ICP pediatric patients are presented in Fig. 3.
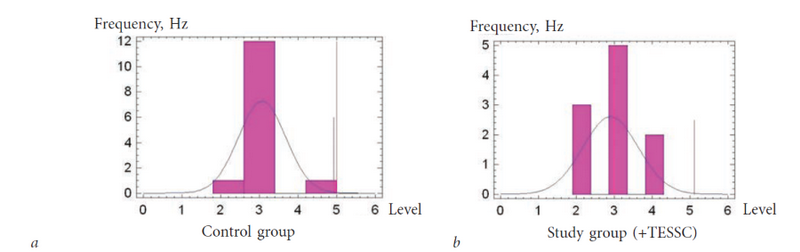
Fig. 3. Levels of motor function in ICP patients on the GMFCS scale: a) control group; b) study group
Degree of muscle spasticity of the lower limbs in the majority of patients amounted to 1–2 points on the Ashworth scale. This value also remained constant during the study owing to the low sensitivity to short courses of rehabilitation. The findings of the evaluations are shown in Fig. 4.

Fig. 4. Degree of spasticity of the ICP patients on the Ashworth scale: a) control group; b) study group
The findings presented in Fig. 3 and 4 show that the study and control groups were clinically homogeneous: differences on the GMFCS (3.2 ± 0.17 and 2.9 ± 0.23, respectively) and degree of spasticity (1.8 ± 0.21 and 1.6 ± 0.22 points, respectively) were not statistically significant (p > 0.05).
Changes in gross motor functions, evaluated on the GMFM-88 scale, were observed during the study in the majority of patients. The results are presented in Fig. 5.

Fig. 5. Changes in gross motor functions after treatment in patients with infantile cerebral paralysis evaluated according to the GMFM-88 scale
Data in Fig. 5 show that 90% of patients in the study group had positive motor function results, measured on the GMFM-88 scale, at the end of the course of robotic mechanotherapy in combination with TESSC. The average score had increased by 9.9 ± 4.3%, measured on the GMFM-88 scale, at the end of the course of rehabilitation. Thus, for patients in this group, positive dynamics, consisting of an increase in the average value of motor functions on the GMFM-88 scale, were evident. In the control group, only 50% of patients had positive outcomes in the assessment of motor functions on the GMFM-88 scale at the end of the course of robotic mechanotherapy. The increase in the average value of lower extremity motor functions in this group was insignificant and amounted to only 2.5 ± 0.78%, which was significantly lower than what was observed in the study group (p < 0.05).
Results of the assessment of muscle strength and range of motion in the lower limbs, obtained using the locomotor tests of the “Lokomat” L-FORCE and L-ROM systems, are presented in Tables 1 and 2.
Table 1. Change in the indicators of the L-FORCE test at the end of the course of robotic mechanotherapy
Group | Increase in muscle strength of the lower limbs (% patients) | |||
Flexion of the hip joint | Extension of the hip joint | Flexion of the knee joint | Extension of the knee joint | |
Study | 60 | 45 | 70 | 55 |
Control | 36 | 36 | 33 | 36 |
Table 2. Range of motion in the hip and knee joints using the L-ROM test before and after the course of robotic mechanotherapy
Group | Range of motion in the lower limb joints (degrees) | |||||||
Left hip joint | Right hip joint | Left knee joint | Right knee joint | |||||
Normal limits | 51 ± 6 | 50 ± 20 | ||||||
Before | After | Before | After | Before | After | Before | After | |
Study | 25 ± 12 | 34 ± 13* | 24 ± 10 | 35 ± 15* | 15 ± 9 | 22 ± 16* | 13 ± 8 | 18 ± 15* |
Control | 25 ± 10 | 33 ± 11* | 25 ± 13 | 34 ± 12* | 22 ± 14 | 25 ± 15 | 21 ± 15 | 21 ± 14 |
Note: * p < 0.05 denotes a significant difference between the conditions before and after.
Thus, according to the study results, a significant increase (Mann–Whitney U test, p < 0.05) in the range of motion was observed in the right and left hip joints in both the study and control groups after the course of rehabilitation; for the right hip joint, this increase was greater in the study group than that in the control group. A significant (p < 0.05) increase in the range of motion in the left and right knee joints was observed only in the study group.
Assessment of muscle activity
Data from both groups show that, in almost all studied muscles, before the onset of rehabilitation, the relative duration of active state of muscles was significantly increased compared with that in healthy children (Fig. 7). In the study group (with TESSC), a significant decrease in the duration of active state towards normalization was observed in the biceps and the anterior tibial muscles after rehabilitation, while an increase was observed in all muscles in the control group. Comparative assessment of the change in the average relative duration of active state of muscles before and after medical procedures is presented in Fig. 7. Because increased muscle activity in pediatric patients with ICP is associated with spasticity, positive changes in the values of this parameter are indicated by a reduction of the relative duration of active muscle state. In the control group, negative changes in this parameter are generally indicated by increase duration of active muscle state after the procedures, i.e., the preservation of the pathological mechanisms of muscle involvement in motor action.
Fig. 6 shows the average values of the relative duration of the active state of each muscle in the study and control groups. Muscle activities before and after the rehabilitation procedures are shown.

Fig. 6. Relative duration of muscle activation in the study (mechanotherapy with TESSC) and control (without TESSC) groups before and after rehabilitation with arbitrary stepping motions in the air. Data on the quadriceps muscle (RF) of the thigh, the biceps muscle (BF) of the thigh, the anterior tibial muscle (TA), the gastrocnemius muscle (G), the right (r) and left (l) legs, are shown. For healthy subjects (NORM), the average values for each muscle in both legs are presented. Statistically significant differences for the changes in relative duration of the active state of muscles before and after treatment are denoted by * p < 0.05

Fig. 7. Changes in relative duration of active state of the muscles in the study and control groups before and after mechanotherapy with independent stepping motions in the air (a) and during walking on the treadmill belt (b). The muscles are marked as in
Thus, examination of the electromyographic activity of muscles in the step cycle revealed that robotic mechanotherapy with TESSC contributes to a change in muscle activity patterns towards normalization, while rehabilitation without TESSC is ineffective, or in some cases, may worsen patient outcomes.
Conclusion
We concluded based on our findings, that motor rehabilitation of ICP pediatric patients using a robotic complex “Lokomat” in combination with TESSC was more effective than rehabilitation with only the “Lokomat” system.
Information on funding and conflict of interest
This work was financially supported by the Ministry of Education and Science of the Russian Federation, with grant № 14.576.21.0020 (unique identifier of the agreement RFMEFI57614X0020).
The authors declare no explicit and potential conflicts of interest with regard to the contents of this article.
About the authors
Galina A. Ikoeva
North-Western State Medical University n.a. I.I. Mechnikov; The Turner Scientific and Research Institute for Children’s Orthopedics
Author for correspondence.
Email: ikoeva@inbox.ru
MD, PhD, assistant professor of the chair of pediatric neurology and neurosurgery Russian Federation
Igor E. Nikityuk
The Turner Scientific and Research Institute for Children’s Orthopedics
Email: femtotech@mail.ru
MD, PhD, leading research associate of the laboratory of physiological and biomechanical research Russian Federation
Olga I. Kivoenko
The Turner Scientific and Research Institute for Children’s Orthopedics
Email: rt-k@yandex.ru
MD, neurologist, head of the rehabilitation department Russian Federation
Tatyana R. Moshonkina
Pavlov Institute of Physiology of the Russian Academy of Sciences
Email: fake@eco-vector.ru
PhD, senior researcher Russian Federation
Irina A. Solopova
Institute for Information Transmission Problems of the Russian Academy of Sciences
Email: fake@eco-vector.ru
PhD, leading researcher Russian Federation
Irina A. Sukhotina
Pavlov Institute of Physiology of the Russian Academy of Sciences
Email: fake@eco-vector.com
PhD, senior researcher Russian Federation
Sergei V. Vissarionov
The Turner Scientific and Research Institute for Children’s Orthopedics; North-Western State Medical University n.a. I.I. Mechnikov
Email: turner01@mail.ru
MD, PhD, professor, Deputy Director for Research and Academic Affairs, head of the department of spinal pathology and neurosurgery.The Turner Scientific and Research Institute for Children’s Orthopedics. Professor of the chair of pediatric traumatology and orthopedics. North-Western State Medical University n.a. I.I. Mechnikov Russian Federation
Valery V. Umnov
The Turner Scientific and Research Institute for Children’s Orthopedics
Email: umnovvv@gmail.com
MD, PhD, professor, head of the department of infantile cerebral palsy Russian Federation
Yurii P. Gerasimenko
Pavlov Institute of Physiology of the Russian Academy of Sciences
Email: fake@eco-vector.com
PhD, professor, corresponding member of RAS, head of laboratory Russian Federation
References
- Бадалян Л.О., Журба Л.Т., Тимонина О.В. Детские церебральные параличи: ДЦП, ЛФK, неврология. — М.: Книга по Требованию, 2013. — 325 с. [Badalyan LO, Zhurba LT, Timonina OV. Detskie tserebral’nye paralichi: DTsP, LFK, nevrologiya. Moscow: Kniga po Trebovaniyu; 2013. 325 p. (In Russ.)]
- Умнов В.В. Нейрохирургические аспекты комплексного ортопедо-нейрохирургического лечения спастических параличей у детей // Вестник Российской военно-медицинской академии. — 2008. — № 1. — С. 87–91. [Umnov VV. Neurosurgical aspects of complex orthopedic and neurosurgical treatment of children with spastic paralysis. Vestnik Rossiyskoy voenno-meditsinskoy akademii. 2008;(1):87-91. (In Russ.)]
- Белова А.Н. Нейрореабилитация: руководство для врачей. — М.: Антидор, 2000. — 566 с. [Belova AN. Neyroreabilitatsiya: rukovodstvo dlya vrachey. Moscow: Antidor; 2000. 566 p. (In Russ.)]
- Вернер Д. Реабилитация детей-инвалидов. — М.: Филантроп, 1995. — 676 с. [Verner D. Reabilitatsiya detey-invalidov. Moscow: Filantrop; 1995. 676 p. (In Russ.)]
- Chung CY, Chen CL, Wong AM. Pharmacotherapy of spasticity in children with cerebral palsy. J Formos Med Assoc. 2011;110:215-222.
- Кожевникова В.Т. Современные технологии в комплексной физической реабилитации больных детским церебральным параличом. — М.: ПБОЮЛ «Т.М. Андреева», 2005. — 238 с. [Kozhevnikova VT. Sovremennye tekhnologii v kompleksnoy fizicheskoy reabilitatsii bol’nykh detskim tserebral’nym paralichom. Moscow: PBOYuL “T.M. Andreeva”; 2005. 238 p. (In Russ.)]
- Боголюбов В.М., Пономаренко Г.Н. Общая физиотерапия. — М.: Медицина, 2003. — 430 с. [Bogolyubov VM, Ponomarenko GN. Obshchaya fizioterapiya. Moscow: Meditsina; 2003. 430 p. (In Russ.)]
- Лильин Е.Т., Доскин В.А. Детская реабилитология. — М.: Литтера, 2011. — 380 с. [Lil’in ET, Doskin VA. Detskaya reabilitologiya. Moscow: Littera; 2011. 380 p. (In Russ.)]
- Черникова Л.А., Клочков А.С. Влияние тренировок на роботизированной системе Lokomat на мобильность при ходьбе у больных с постинсультными гемипарезами // Вопросы курортологии, физиотерапии и лечебной физической культуры. — 2014. — Т. 91. — Вып. 3. — С. 13–17. [Chernikova LA, Klochkov AS. The influence of physical training with the use of a Lokomat robotic system on the walking ability of the patients presenting with post-stroke hemiparesis. Voprosy kurortologii, fizioterapii i lechebnoy fizicheskoy kul’tury. 2014;91(3):13-17. (In Russ.)]
- Johnston TE, Watson KE, et al. Effects of a supported speed treadmill training exercise program on impairment and function for children with cerebral palsy. Dev Med Child Neurol. 2011;53(8):742-750.
- Smania N, Bonetti P, Gandolfi M, et al. Improved gait after repetitive locomotor training in children with cerebral palsy. Am J Phys Med Rehabil. 2011;90(2):137-149. doi: 10.1097/phm.0b013e318201741e.
- Икоева Г.А., Кивоенко О.И., Полозенко О.Д. Роботизированная механотерапия в реабилитации детей с церебральным параличом после комплексного ортопедо-хирургического лечения // Нейрохирургия и неврология детского возраста. — 2012. — №. 4. — С. 32–36. [Ikoeva GA, Kivoenko OI, Polozenko OD. Use of robot-drive mechanotherapy in children with the cerebral palsy after complex ortopedical and surgical treatment. Pediatric Neurosurgery and Neurology. 2012;(4):32-36. (In Russ.)]
- Gorodnichev RM, Pivovarova EA, Puhov A, et al. Transcutaneous electrical stimulation of the spinal cord: a noninvasive tool for the activation of stepping pattern generators in humans. Human Physiology. 2012;38(2):158-167. doi: 10.1134/S0362119712020065.
- Xu KS, He L, Li JL, Mai JN. Effects of transcutaneous electrical nerve stimulation on motor function in ambulant children with spastic cerebral palsy: a randomized trial. Zhonghua Er Ke Za Zhi. 2007;45(8):564.
- Икоева Г.А., Кивоенко О.И., Мошонкина Т.Р., и др. Сравнительный анализ эффективности двигательной реабилитации детей с церебральным параличом с использованием роботизированной механотерапии и чрескожной электрической стимуляции спинного мозга // Международный журнал прикладных и фундаментальных исследований. — 2016. — № 2 (часть 2). — С. 200–203. [Ikoeva GA, Kivoenko OI, Moshonkina TR. Comparative analysis of the efficiency of the motor rehabilitation in children with cerebral palsy using robotic mechanotherapy and transcutaneos electrical stimulation of the spinal cord. Mezhdunarodnyj zhurnal prikladnyh i fundamental’nyh issledovanij. 2016;2(2):200-203. (In Russ.)]
- Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67(2):206-207.
- Russell DJ, Rosenbaum PL, Cadman DT, et al. The gross motor function measure: a means to evaluate the effects of physical therapy. Dev Med Child Neurol. 1989;31(3):341-352. doi: 10.1111/j.1469-8749.1989.tb04003.x.
- Богачева И.Н., Мошонкина Т.Р., Боброва Е.В., и др. Эффект чрескожной электрической стимуляции спинного мозга и механотерапии в регуляции активности мышц ног // Вестник Тверского государственного университета. Сер. «Биология и экология». — 2015. — № 2. — С. 7–17. [Bogacheva IN, Moshonkina TR, Bobrova EV, et al. Effect of transcutaneous electrical spinal cord stimulation and mechanotherapy in the muscle activity regulation. Vestnik Tverskogo gosudarstvennogo universiteta. Ser. Biologiya i ekologiya. 2015;(2):7-17. (In Russ.)]
- Stackhouse SK, Binder-Macleod SA, Lee SCK. Voluntary muscle activation, contractile properties, and fatigability in children with and without cerebral palsy. Muscle and Nerve. 2005;31(5):594-601. doi: 10.1002/mus.20302.
Supplementary files










