Осложнение места доступа при интракардиальных вмешательствах
- Авторы: Кимков А.В.1
-
Учреждения:
- Госпиталь св. Катарины
- Выпуск: Том 2, № 1 (2022)
- Страницы: 5-12
- Раздел: Обзоры
- URL: https://journals.eco-vector.com/cardar/article/view/100389
- DOI: https://doi.org/10.17816/cardar100389
- ID: 100389
Цитировать
Аннотация
В последние десятилетия многократно возросло количество интракардиальных процедур с использованием чреcкожного пункционного доступа. Несмотря на приобретение операторами экспертизы и стандартизации методов, проблема осложнений остается актуальной.
Цель: проанализировать частоту и характер осложнений чрезкожного доступа при интракардиальных вмешательствах. Предложить рекомендации для снижения частоты осложнений.
Материалы и методы. Анализ данных, опубликованных в международных рецензируемых журналах по теме, а также опыта клиники сосудистой хирургии госпиталя св. Катарины.
Выводы. Частота и тяжесть осложнений зависят от опыта оператора, размера и частоты смены инструмента, а также соблюдения правил предоперационной диагностики и послеоперационного ведения пациента.
Рекомендации. Стандартизированная предоперационная подготовка, тщательное планирование вмешательства, анализ состояния сосудов доступа, соблюдение правил пункции сосуда и грамотное выполнение послеоперационной компрессии в сочетании с применением ушивающих устройств по показаниям могут снизить частоту и тяжесть осложнений.
Ключевые слова
Полный текст
В 1929 году немецкий уролог W. Forsmann ввел сам себе в кубитальную вену мочевой катетер и протолкнул его в центральном направлении. После этого он сделал рентгенографический снимок грудной клетки в прямой проекции. Это событие стало первым доказанным случаем катетеризации полости сердца [1]. Впоследствии, в 1956 году, автору была присуждена Нобелевская премия по медицине. В 1953 году шведский радиолог S.I. Seldinger предложил технику пункции сосуда для последующих манипуляций, названную его именем [2]. И наконец, в 1990-е годы F. Kiemenej и G.J. Laarman разработали и внедрили в практику технологию трансрадиальной чреcкожной коронарной интервенции [3]. В настоящее время интервенционные технологии являются основой многих специальностей.
В 2018 году в РФ выполнено 220 тыс. коронарных стентирований, их число увеличилось за 10 лет почти в 4 раза. В США в 2017 году было выполнено 75 тыс. предсердных абляций [4]. Во всех случаях манипуляции выполнялись либо через трансрадиальный, либо через трансфеморальный доступ. Дискуссия о преимуществах одного доступа перед другим завершилась после публикации ряда исследований, и прежде всего исследования RIVAL [5]. В настоящий момент предпочтительным является радиальный доступ при подходящей анатомии и отсутствии особых противопоказаний.
Несмотря на накопленный громадный опыт, большой проблемой остаются осложнения места доступа при проведении интервенционных процедур. Еще в 90-е годы у 6% всех пациентов после коронарных интервенций развивались осложнения. Из них в 22–25% случаев требовалась гемотрансфузия, а в 21–38% ― хирургическое вмешательство [6, 7]. В настоящее время, с накоплением опыта, частота осложнений существенно снизилась (до 1–2%) [8–11], но, с учетом огромного количества вмешательств, не потеряла своей актуальности. Так, например, смертность у пациентов, переживших такое осложнение, составляет 7,5% (без осложнений ― 1,1%), продолжительность пребывания в стационаре ― вдвое выше, а стоимость стационарного лечения ― почти вдвое выше ($9,583 vs. $18 350) [12].
Основными осложнениями места доступа являются: гематома, ишемия зависимой от сосуда доступа конечности, кровотечение (в том числе гемодинамически значимое), ложная аневризма с необходимостью ревизии, эвульсия сосуда, инфекция. Не следует упускать из виду социально-экономические и психологические проблемы (отвлечение персонала, психологическая травма пациента, блокирование пациента и избыточное использование ресурсов). Факторами, повышающими риск развития осложнений, являются женский пол, возраст старше 65, площадь поверхности тела > 1,62 м2, большая продолжительность процедуры, высокое место пункции при трансфеморальном доступе, наличие в анамнезе геморрагического диатеза, ожирение, неконтролируемая артериальная гипертензия, почечная недостаточность (уровень креатинина > 2 мг/д), применение ингибиторов гликопротеина IIB/IIIA, а также периферический атеросклероз [8–14].
Осложнения лучевого доступа
Окклюзия лучевой артерии ― наиболее частое осложнение при соответствующем доступе. Оно регистрируется в 2–18% всех трансрадиальных интервенций. Его возникновение зависит во многом от опыта оператора, способа компрессии и, главное, качества его применения. По данным исследований, течение этого осложнения, как правило, бессимптомное и специального лечения не требует. В основе патогенетического процесса лежит тромбообразование на участках поврежденной интимы. Симптоматическое течение окклюзия лучевой артерии принимает только в случае несостоятельности пальмарных анастомозов или наличия окклюзии локтевой артерии. В этой ситуации проведение перед исследованием теста Эллена (Allen test) поможет предотвратить развитие серьезного осложнения. Проведение этого теста технически несложно и не требует привлечения дополнительных ресурсов. В ситуации окклюзии обеих артерий пациенту угрожает ишемия зависимого от этих сосудов региона и, при неблагоприятном течении ― развитие некрозов и необходимость ампутации. Кроме того, периоперативная инъекция 5 000 МЕ гепарина снижает риск окклюзии сосуда (71 vs. 4,3%). Факторами повышающими риск окклюзии лучевой артерии являются: избыточная послеоперационная компрессия, повторные пункции и большой диаметр шлюза.
К группе неокклюзивных поражений лучевой артерии относятся, прежде всего, гиперплазия интимы и сосудистый ремоделлинг. Исследования послеоперационного состояния интимы с использованием внутрисосудистого ультразвукового сканирования показали существенную гиперплазию интимы с результирующим значительным уменьшением диаметра последнего. Небольшая серия с использованием оптической когерентной томографии выявила наличие разрывов интимы у существенной группы пациентов (67%) и диссекцию медии (36%). Эти повреждения приводят к снижению качества сосуда при его последующем использовании в качестве графта при артериальной реваскуляризации миокарда. Ретроспективная серия показала снижение ранней проходимости таких графтов.
Спазм лучевой артерии наблюдается в 5–10% всех интервенций. Он редко приводит к серьезным последствиям, однако может обусловить неудачу самой процедуры. Факторы риска: малый диаметр сосуда, женский пол, многократная смена катетера, использование длинного шлюза, а также неопытность оператора. Лучевая артерия обладает хорошо выраженной tunica media, которая находится под контролем α-1 адренорецепторов. В профилактических целях можно применять седацию пациента и местную анестезию с целью контроля эффекта циркулирующих эндогенных катехоламинов. Спазм хорошо поддается лечению. В настоящее время применяются нитроглицерин, подкожные инъекции лидокаина и разного рода вазодилатационные коктейли. Снижению риска развития спазма способствует также использование низкопрофильных гидрофильных шлюзов [25, 26]. А. Ouadhour et al. на основании собственного исследования предполагают, что подкожное введение 0,5 мг динитрата изосорбида с 1% лидокаина для местной анестезии может улучшить функцию лучевого доступа [27].
Перфорация сосуда ― редкое осложнение, которое, однако, может повлечь за собой тяжелые последствия в виде потери доступа, необходимости конверсии, а в случае несвоевременной реакции ― развития гематомы, компартмент-синдрома и ишемии зависимой области. В анализе, проведенном R.A. Calvino-Santos et al., сообщается о частоте его наступление реже чем в 1% случаев. Приведенная в больших сериях статистика показывает частоту ниже 0,1% [6]. Причем абсолютное большинство этих осложнений наступило у низкорослых женщин с извитыми сосудами предплечья [30]. Безопасность доступа у таких пациентов может быть повышена при использовании длинного шлюза [30] и проводникового катетера [31]. При раннем обнаружении перфорации возможно использование периферического баллона малого диаметра для ангиопластики [32].
Компартмент-синдром ― намного более редкое, но грозное осложнение, требующее экстренной фасциотомии. Его частота описана в больших сериях наблюдений на уровне 0,004% [33]. Диагноз ставится на основе клинических проявлений в виде массивной опухоли предплечья с симптоматикой периферической ишемии, прежде всего ― неврологических нарушений.
Ложная аневризма ― еще одно редкое осложнение. Возникает менее чем 0,1% случаев [6]. Механизм возникновения ― пенетрирующая травма сосуда, которая не была своевременно распознана. Развитию ложной аневризмы способствуют также многократные пункции сосуда и некорректное применение компрессии места доступа (например, TR-Band) после окончания процедуры. Диагноз ставится с помощью ультразвукового исследования. Лечение: консервативное (адекватная компрессия, инъекция тромбина в полость ложной аневризмы под контролем УЗИ [34, 35]) либо хирургическое (ревизия места доступа с эвакуацией гематомы и наложением шва). Лигатура лучевой артерии требуется крайне редко.
В таблице 1 представлены наиболее частые осложнения радиального доступа, их частота и методы профилактики и лечения.
Таблица 1. Осложнения трансрадиального доступа (модифицировано по Kanei)
Осложнение | Частота | Факторы риска | Профилактика и лечение |
Окклюзия | 2–18% | Избыточная компрессия Повторные пункции Большой диаметр шлюза | Антикоагуляция Качественный гемостаз |
Неокклюзивное поражение лучевой артерии | Часто | Критическая оценка при использовании в качестве графта | |
Ишемия кисти | Крайне редко | Продолжительное канюлирование | Оценка кровообращения до процедуры |
Спазм | 5–10% | Малый диаметр сосуда Женский пол Многократная смена катетера Большой диаметр шлюза Малый опыт | Спазмолитическая терапия Бережная манипуляция |
Перфорация | 0,1% | Агрессивные манипуляции Избыточная антикоагуляция | Своевременный диагноз и бандажирование |
Ложная аневризм | < 0,1% | Многократные пункции Бактериальное обсеменение катетера Избыточная антикоагуляция Большой диаметр шлюза | Компрессия Инъекция тромбина Бандажирование |
Поражение нерва | Крайне редко | Многократные пункции | Неврологическая терапия |
Артериовенозная фистула | Крайне редко | Многочисленный пункции | Хирургическая коррекция при необходимости |
Значимое кровотечение | 0,15% | Корректное проведение гемостаза Трансфузия Хирургическое лечение при необходимости |
Осложнения трансфеморального доступа
Несмотря на доказанные преимущества трансрадиального доступа, трансфеморальный доступ продолжает применяться достаточно широко при наличии показаний или как предпочтительный для конкретного оператора. Основными осложнениями при данном доступе являются гематома места пункции, ретроперитонеальная гематома, ложная аневризма, артериовенозная фистула, а также артериальная диссекция, лимитирующая кровоток. Намного реже встречаются инфекции, тромбоз, продолжительные повреждения нервных структур. Громадную роль в возникновении этих осложнений играет некорректное выполнение пункции сосуда. Многие интервенционные специалисты не имеют практического хирургического опыта и часто отклоняются от рекомендованной техники пункции, считая ее малозначительной стадией вмешательства.
При этом анатомия общей бедренной артерии отличается малой вариабельностью, выражающейся в разной высоте бифуркации. Общей рекомендацией является пункция под контролем УЗИ. При выполнении пункции под радиологическим контролем следует ориентироваться на головку бедренной кости и пунктировать слегка медиальнее ее центра в направлении к мечевидному отростку под углом 45°. Схематически анатомия общей бедренной артерии представлена на рисунке 1.
Рис. 1. Топография бедренной артерии (из [36])
Не следует выполнять пункцию выше паховой связки, так как в этой области наружная подвздошная артерия ориентирована в передне-заднем направлении, и пункционная канюля проходит параллельно сосуду, не обеспечивая пункцию. Кроме того, при выполнении высокой пункции отсутствует костное основание для выполнения послеоперационной компрессии. В связи с этим существенно возрастает риск кровотечения с образованием ретроперитонеальной гематомы.
Следующие факторы следует отнести к факторам риска возникновения осложнения:
- недостаточное знание анатомии бедренной артерии,
- малый опыт оператора,
- повышенный индекс массы тела,
- женский пол,
- отказ от использования ультразвуковой навигации,
- беспокойный пациент.
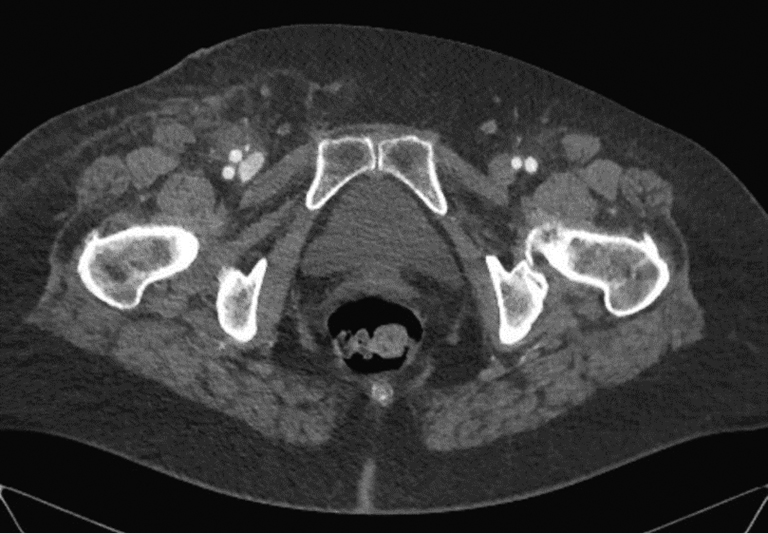
Гематома места пункции ― наиболее частое осложнение, возникающее, по данным разных авторов, в 5–23% случаев. Масштабы гематомы варьируют от незначительного окрашивания кожи в месте пункции до массивной опухоли, требующей хирургического лечения и гемотрансфузии. Причинами возникновения гематомы можно считать, прежде всего, многократные попытки пункции, некорректную или недостаточную по времени послеоперационную компрессию, а также некорректное применение ушивающих устройств. В абсолютном большинстве случаев гематомы не требуют специального лечения и систируют в течение 1–2 нед. Наблюдение и локальная терапия гепариновой мазью вполне достаточны. При детектировании нарастающей гематомы во время самого вмешательства возможно применение эндоваскулярных методов лечения, например баллонная компрессия или имплантация короткого стента/стент-протеза. В этом случае необходимо выполнение контралатерального доступа. Если возникает необходимость имплантации стента либо стент-графта, следует принимать во внимание, что место пункции находится кпереди от тазобедренного сустава, то есть находится в подвижном сегменте. Имплантация стента в таком сегменте несет в себе риск перелома имплантата и, как следствие, возникновения обструкции сосуда, лимитирующей кровоток. Иногда размеры гематомы требуют хирургического лечения. Компьютерная томограмма такой гематомы представлена на рисунке 2. В случае быстрого развития массивной гематомы, не исключены некротические изменения мягких тканей. Пример такого случая представлен на рисунке 4. Особым вариантом местной гематомы является возникновение ложной аневризмы (0,5–9%). Здесь показано экстренное хирургическое лечение.
Рис. 2. Массивная гематома паховой области и бедра справа после чрескожного коронарного вмешательства (наблюдение автора)
Рис. 3. Массивная гематома места пункции, распространившаяся на проксимальный отдел бедра: а ― радиологическая картина, b ― стремительный некроз мягких тканей из-за давления гематомы изнутри, c ― клиническая картина через 8 дней после эвакуации гематомы и ревизии места пункции (наблюдение автора)
Рис. 4. Гигантское лимфоцеле (>2,5 л), возникшее после эвакуации забрюшинной гематомы (наблюдение автора)
При выполнении послеоперационной компрессии следует учитывать размер инструмента, использовавшегося при катетеризации сосуда. Усилие компрессии должно быть приблизительно на 20 мм Hg выше системного систолического давления. Для определения времени компрессии можно пользоваться ориентирами, представленными в таблице 2. При использовании инструментов диаметром более 6Fr для артериального доступа рекомендуется применение ушивающего устройства. В раннем послеоперационном периоде важно строгое соблюдение пациентом постельного режима и контроль места пункции персоналом отделения.
Таблица 2. Рекомендуемое время компрессии и постельного режима при трансфеморальном артериальном доступе
Инструмент | Время компрессии, мин | Постельный режим, ч |
Катетер 4F | 5 | 2–4 |
Шлюз 4F Катетер 5F | 10–15 | 6–8 |
Шлюз 5F | 15–20 | 6–8 |
Шлюз 6F | > 20 | > 8 |
Ретроперитонеальная гематома ― редкое, но грозное осложнение. Встречается с частотой 0,8–0,44%. Возникновение данной патологии связано, прежде всего, с супраингвинальной пункцией наружной подвздошной артерии. Основная опасность заключается в отложенном проявлении симптоматики, которая проявляется гиповолемией, болями в ипсилатеральном гипогастрии и ухудшении общего состояния пациента. Диагноз ставится на основе физикального обследования, ультразвукового сканирования и КТ с контрастным веществом. Лечение ― экстренная ревизия с наложением сосудистого шва, эвакуацией гематомы и наложением дренажа в забрюшинном пространстве. В ряде случаев требуется имплантация интерпоната и гемотрансфузия. В редких случаях возникают рецидивы кровотечения или развитие лимфоцеле (рис. 4).
Артериовенозная фистула (0,2–2,1%) возникает при акцидентальной пункции артерии через вену либо вены через артерию (рис. 5). В абсолютном большинстве случаев такие фистулы закрываются самостоятельно в течение нескольких недель. Хирургическое лечение показано в случае появления признаков перегрузки правых отделов сердца.
Рис. 5. Постинтервенционная артериовенозная фистула. Стрел-ка указывает на контрастирование бедренной вены (наблюде-ние автора)
Дома:
- Перед проведением пункции лучевой артерии проведите тест Эллена.
- Контролируйте положение инструмента в просвете сосуда.
- Избегайте частой, неоправданной смены инструмента.
- Проводите адекватный гемостаз.
- При применении ушивающих устройств соблюдайте инструкцию.
- Грамотно проводите мануальную компрессию.
- Убеждайте пациентов соблюдать постельный режим.
- Контролируйте место доступа перед выпиской.
- Передавайте опыт.
Об авторах
Александр Вадимович Кимков
Госпиталь св. Катарины
Автор, ответственный за переписку.
Email: alexandre04@inbox.ru
ORCID iD: 0000-0002-1774-938X
д-р мед. наук
Германия, Кёльн-ФрехенСписок литературы
- Radner S. Thoracal aortography by catheterization from the radial artery; preliminary report of a new technique // Acta radiol. 1948. Vol. 29, No. 2. P. 178–180. doi: 10.3109/00016924809132437
- Seldinger S.I. Catheter replacement of the needle in percutaneous arteriography; a new technique // Acta radiol. 1953. Vol. 39, No. 5. P. 368–376. doi: 10.3109/00016925309136722
- Kiemeneij F., Laarman G.J. Percutaneous transradial artery approach for coronary stent implantation // Cathet Cardiovasc Diagn. 1993. Vol. 30, No. 2. P. 173–178. doi: 10.1002/ccd.1810300220
- Campeau L. Percutaneous radial artery approach for coronary angiography // Cathet Cardiovasc Diagn. 1989. Vol. 16, No. 1. P. 3–7. doi: 10.1002/ccd.1810160103
- Mansour M., Karst E., Heist E.K., et al. The Impact of First Procedure Success Rate on the Economics of Atrial Fibrillation Ablation // JACC: Clinical Electrophysiology. 2017. Vol. 3, No. 2. P. 129–138. doi: 10.1016/j.jacep.2016.06.002
- Byrne R.A., Cassese S., Linhardt M., Kastrati A. Vascular access and closure in coronary angiography and percutaneous intervention // Nat Rev Cardiol. 2013. Vol. 10. P. 27–40. doi: 10.1038/nrcardio.2012.160
- Waksman R., King S.B. III, Douglas J.S., et al. Predictors of groin complications after balloon and new-device coronary intervention // Am J Cardiol. 1995. Vol. 75, No. 14. P. 886–889. doi: 10.1016/s0002-9149(99)80681-x
- Omoigui N.A., Califf R.M., Pieper K., et al. Peripheral vascular complications in the Coronary Angioplasty Versus Excisional Atherectomy Trial (CAVEAT-I) // J Am Coll Cardiol. 1995. Vol. 26, No. 4. P. 922–930. doi: 10.1016/0735-1097(95)00263-4
- Piper W.D., Malenka D.J., Ryan T.J. Jr., et al. Predicting vascular complications in percutaneous coronary interventions // Am Heart J. 2003. Vol. 145, No. 6. P. 1022–1029. doi: 10.1016/S0002-8703(03)00079-6
- Dauerman H.L., Applegate R.J., Cohen D.J. Vascular closure devices: the second decade // J Am Coll Cardiol. 2007. Vol. 50, No. 17. P. 1617–1626. doi: 10.1016/j.jacc.2007.07.028
- Marso S.P., Amin A.P., House J.A., et al. Association between use of bleeding avoidance strategies and risk of periprocedural bleeding among patients undergoing percutaneous coronary intervention // JAMA. 2010. Vol. 303, No. 21. P. 2156–2164. doi: 10.1001/jama.2010.708
- Tavris D.R., Gallauresi B.A., Lin B., et al. Risk of local adverse events following cardiac catheterization by hemostasis device use and gender // J Invasive Cardiol. 2004. Vol. 16, No. 9. P. 459–464.
- Romaguera R., Wakabayashi K., Laynez-Carnicero A., et al. Association between bleeding severity and long-term mortality in patients experiencing vascular complications after percutaneous coronary intervention // Am J Cardiol. 2012. Vol. 109, No. 1. P. 75–81. doi: 10.1016/j.amjcard.2011.08.007
- Sanmartin M., Gomez M., Rumoroso J.R., et al. Interruption of blood flow during compression and radial artery occlusion after transradial catheterization // Catheter Cardiovasc Interv. 2007. Vol. 70, No. 2. P. 185–189. doi: 10.1002/ccd.21058
- Pancholy S.B. Transradial access in an occluded radial artery: new technique // J Invasive Cardiol. 2007. Vol. 19, No. 12. P. 541–544.
- Spaulding C., Lefevre T., Funck F., et al. Left radial approach for coronary angiography: results of a prospective study // Cathet Cardiovasc Diagn. 1996. Vol. 39, No. 4. P. 365–370. doi: 10.1002/(SICI)1097-0304(199612)39:4<365::AID-CCD8>3.0.CO;2-B
- Sakai H., Ikeda S., Harada T., et al. Limitations of successive transradial approach in the same arm: the Japanese experience // Catheter Cardiovasc Interv. 2001. Vol. 54, No. 2. P. 204–208. doi: 10.1002/ccd.1268
- Saito S., Ikei H., Hosokawa G., Tanaka S. Influence of the ratio between radial artery inner diameter and sheath outer diameter on radial artery flow after transradial coronary intervention // Catheter Cardiovasc Interv. 1999. Vol. 46, No. 2. P. 173–178. doi: 10.1002/(SICI)1522-726X(199902)46:2<173::AID-CCD12>3.0.CO;2-4
- Wakeyama T., Ogawa H., Iida H., et al. Intima-media thickening of the radial artery after transradial intervention. An intravascular ultrasound study // J Am Coll Cardiol. 2003. Vol. 41, No. 7. P. 1109–1114. doi: 10.1016/s0735-1097(03)00089-5
- Yonetsu T., Kakuta T., Lee T., et al. Assessment of acute injuries and chronic intimal thickening of the radial artery after transradial coronary intervention by optical coherence tomography // Eur Heart J. 2010. Vol. 31, No. 13. P. 1608–1615. doi: 10.1093/eurheartj/ehq102
- Kamiya H., Ushijima T., Kanamori T., et al. Use of the radial artery graft after transradial catheterization: is it suitable as a bypass conduit? // Ann Thorac Surg. 2003. Vol. 76, No. 5. P. 1505–1509. doi: 10.1016/s0003-4975(03)01018-x
- Kiemeneij F. Prevention and management of radial artery spasm // J Invasive Cardiol. 2006. Vol. 18, No. 4. P. 159–160.
- Coppola J., Patel T., Kwan T., et al. Nitroglycerin, nitroprusside, or both, in preventing radial artery spasm during transradial artery catheterization // J Invasive Cardiol. 2006. Vol. 18, No. 4. P. 155–158.
- Ouadhour A., Sideris G., Smida W., et al. Usefulness of subcutaneous nitrate for radial access // Catheter Cardiovasc Interv. 2008. Vol. 72, No. 3. P. 343. doi: 10.1002/ccd.21645
- Sanmartin M., Cuevas D., Goicolea J., et al. Complicaciones vasculares asociadas al acceso transradial para el cateterismo cardiaco // Rev Esp Cardiol. 2004. Vol. 57, No. 6. P. 581–584. doi: 10.1016/S0300-8932(04)77150-X
- Calvino-Santos R.A., Vazquez-Rodriguez J.M., Salgado-Fernandez J., et al. Management of iatrogenic radial artery perforation // Catheter Cardiovasc Interv. 2004. Vol. 61, No. 1. P. 74–78. doi: 10.1002/ccd.10698
- Gunasekaran S., Cherukupalli R. Radial artery perforation and its management during PCI // J Invasive Cardiol. 2009. Vol. 21, No. 2. P. E24–26.
- Rigatelli G., Dell'Avvocata F., Ronco F., Doganov A. Successful coronary angioplasty via the radial approach after sealing a radial perforation // JACC Cardiovasc Interv. 2009. Vol. 2, No. 11. P. 1158–1159. doi: 10.1016/j.jcin.2009.05.026
- Tizon-Marcos H., Barbeau G.R. Incidence of compartment syndrome of the arm in a large series of transradial approach for coronary procedures // J Interv Cardiol. 2008. Vol. 21, No. 5. P. 380–384. doi: 10.1111/j.1540-8183.2008.00361.x
- Kang S.S., Labropoulos N., Mansour M.A., et al. Expanded indications for ultrasound-guided thrombin injection of pseudoaneurysms // J Vasc Surg. 2000. Vol. 31, No. 2. P. 289–298. doi: 10.1016/s0741-5214(00)90160-5
- Liou M., Tung F., Kanei Y., Kwan T. Treatment of radial artery pseudoaneurysm using a novel compression device // J Invasive Cardiol. 2010. Vol. 22. P. 293–295.
- Kanei Y., Kwan T., Nakra N.C., et al. Transradial cardiac catheterization: A Review of Access Site Complications // Catheter Cardiovasc Interv. 2011. Vol. 78, No. 6. P. 840–846. doi: 10.1002/ccd.22978
- Levine G.N., Bates E.R., Blankenship J.C., et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions // J Am Coll Cardiol. 2011. Vol. 58, No. 24. P. e44–122. doi: 10.1016/j.jacc.2011.08.007
- Sherev D.A., Shaw R.E., Brent B.N. Angiographic predictors of femoral access site complications: implication for planned percutaneous coronary intervention // Catheter Cardiovasc Interv. 2005. Vol. 65, No. 2. P. 196–202. doi: 10.1002/ccd.20354
- Ben-Dor I., Sharma A., Rogers T., et al. Micropuncture technique for femoral access is associated with lower vascular complications compared to standard needle // Catheter Cardiovasc Interv. 2021. Vol. 97, No. 7. P. 1379–1385. doi: 10.1002/ccd.29330
- Larena-Avellaneda A., Kölbel T., Carpenter S.W., et al. Iatrogene Verletzungen an den Zugangsgefäßen für intravaskuläre Prozeduren // Notfall + Rettungsmedizin. 2017. Vol. 20. P. 305–314. doi: 10.1007/s10049-017-0287-5
Дополнительные файлы