Pathological changes of the cervical spine in children with cervical pain syndrome
- 作者: Bakhteeva N.K.1, Ionova T.A.2, Belonogov V.N.3, Bazhanov S.P.2, Ostrovskij V.V.2
-
隶属关系:
- Saratov State Medical University n.a. V.I.Razumovsky
- Saratov Scientific and Research Institute of Traumatology and Orthopedics
- Saratov State Medical University n.a. V.I. Razumovsky
- 期: 卷 4, 编号 4 (2016)
- 页面: 12-20
- 栏目: Articles
- ##submission.dateSubmitted##: 09.01.2017
- ##submission.dateAccepted##: 09.01.2017
- ##submission.datePublished##: 14.12.2016
- URL: https://journals.eco-vector.com/turner/article/view/5891
- DOI: https://doi.org/10.17816/PTORS4412-20
- ID: 5891
如何引用文章
详细
Background. Interpretation of cervical pain syndrome in children is complicated, resulting in delayed diagnosis of developmental juvenile osteochondrosis. Thus, updating the diagnostic methods of this pathology is particularly important.
Aim. To improve methods of cervical spine diagnostics in children with cervical pain syndrome at the base of vertebral and basilar arteries using duplex ultrasound.
Material and methods. The study cohort included 148 pediatric patients aged 4–18 years who were divided into two groups: a treatment group of 108 children with cervical pain syndrome and a control group of 40 healthy children. Clinical, radiological (ultrasound, X-ray, and MRI), and statistical methods were used for comparisons.
Results. Duplex ultrasound of 108 patients revealed pathological changes of qualitative and quantitative features of C- or S-shaped, corner bend, mesh, excessive, and wave-shaped tortuosity deformities, as well as a reduction or expanse in diameter of one or two of the spinal arteries (SAs). The absence of an influence of osseous cervical spine structures on SAs was considered a sign of congenital genesis of SA deformity, while segmental instability of C2-C3 and/or C3-C4, atlanto-axial subluxation, and a Kimmerle anomaly were considered signs of extravascular compression of SAs. Regardless of the deformity genesis, blood flow was deficit in the vertebral basilar basin because of local hemodynamic disorders at the site of the deformity, particularly in older children. MRI revealed signs of intervertebral disc hypohydration at C2-C3 and/or C3-C4.
Conclusion. Pathological changes in SAs of both congenital and acquired genesis resulted in hemodynamic disorders in the vertebral basilar basin in children with cervical pain syndrome, particularly older children.
全文:
Introduction
The prevalence of cervical pain in children ranges from 5 to 72%, and its rate of occurrence tends to increase [1, 2]. In most cases, the complaints of discomfort in the neck, headaches, and dizziness are considered by neurologists and pediatricians as the manifestation of vegetovascular dystonia. However, these symptoms can be caused by functional and organic changes in the bone and soft-tissue structures of the cervical spine [3–6]. The increased incidence of cervical pain in children and the simultaneous complexity of this interpretation lead to late diagnosis of the developing juvenile osteochondrosis, which decreases the patient’s quality of life. In this regard, the use of modern research methods to improve the diagnosis of cervical spine diseases in children with cervical pain syndrome acquires special significance [7–11].
The aim of this study was to increase the accuracy of diagnosis of cervical spine diseases in children with cervical pain syndrome, including duplex ultrasound of the vertebral and basilar arteries.
Material and methods
The study cohort included 148 pediatric patients aged 4–18 years, of which 108 had cervical pain syndrome (study group) and 40 were healthy patients of the same age (control group). The study cohort consisted of 59 boys and 49 girls. Written informed consent for participation in the study was voluntarily provided by patients or their parents. The study was approved by the local ethics committee of the Federal State Budgetary Institution Saratov Research Institute of Traumatology and Orthopedics of the Russian Federation Ministry of Health (Protocol No. 5 dated 10/29/2009).
During the study the clinical, radiation (ultrasound, X-ray, magnetic resonance tomography), and the statistical research methods were used.
Ultrasonography was performed on the multi-function, high-class ultrasound scanner Technos MPX manufactured by ISAOTE (Italy). The qualitative (diameter, vascular geometry, and the level of entry into the bony canal) and quantitative characteristics of the vertebral artery blood flow were determined (Vps: peak systolic velocity of blood flow; Ved: end diastolic velocity of blood flow; and RI, the resistance index). The hemodynamic status of the vertebral artery (VA) and basilar artery (BA) was represented by the peak systolic velocity of blood flow. X-ray of the cervical spine in the anteroposterior and lateral views was performed using a digital X-ray machine manufactured by Apelem (France). According to the results of duplex ultrasound of the cervical vessels, the necessity for transoral (through the open mouth) X-ray of the cervical spine and functional tests of maximum flexion and extension of the neck was determined. Magnetic resonance imaging (MRI) of the cervical spine was performed to determine the extent of damage to the intervertebral discs when unstable vertebral-motor segments on functional X-ray images were detected. This was performed using a MRI scanner of the open-type Aperto manufactured by Hitachi (Japan), with the magnetic field strength of 0.4 T.
The numerical results were processed using parametric variation statistics with significance determined by Student’s t tests. The results were considered significant at р < 0.05.
Results and discussion
All patients had pain in the neck, headache, and dizziness. During examination, we considered the presence of the natural bend of the head, shoulder girdle asymmetry, contouring, and tension of the shoulder girdle and neck muscles, and painfulness of the paravertebral points and spines of the cervical vertebrae.
Duplex ultrasound of VA was performed in 108 patients with cervical pain. In 94 (87%) of the 108 patients, pathological changes of the qualitative and quantitative characteristics of one or two VA were found, which were used to divide all patients into two groups. In the remaining 14 (13%) patients, the ultrasound VA figures were within normal limits. Considering the localization of pathological changes of the blood vessels, subgroups were formed: a, V1 segment; b, V2 segment; c, V3 segment; d, V1–V4 segments; and e, combination of different segments (Table 1). Subgroup e was excluded from the analysis due to the diversity of localization variants. In the bilateral process, the segmental levels of lesion localization were distributed to the respective subgroups for statistical analysis.
Table 1. The distribution of pediatric patients by subgroups, depending on the level of unilateral or bilateral lesions of the vertebral arteries and the total number of the affected segments
Subgroups | The groups of patients aged 4–18 (n = 94) | The number of affected segments based on pathologic changes in the collateral vertebral artery | |
With unilateral lesion of the vertebral arteries | With bilateral lesion of the vertebral arteries | ||
а | 3 (3,2 %) | 3 (3,2 %) | 9 |
b | 36 (38,3 %) | 16 (17,0 %) | 68 |
c | 11(11,7 %) | 5 (5,3 %) | 21 |
d | – | 5 (5,3 %) | 10 |
e | 6 (6,4 %) | 9 (9,6 %) | – |
Total | 56 (59,6 %) | 38 (40,4 %) | – |
For a duplex study of the VA and BA in the patients of the subgroup а, there was a statistically significant decrease in Vps in segment V1 to 0.28 ± 0.10 m/s (p < 0.003); this increased in segment V2 to 0.69 ± 0.21 m/s (p < 0.05). The rest of the blood flow velocity indices in segments V3 and V4 of the VA and BA did not differ from the control group (Table 2).
Table 2. Blood flow velocity indices of the vertebral arteries and basilar artery in children with C- and S-shaped deformities of the V1 segment of the vertebral artery (n = 9; М ± m)
Blood flow velocity | Groups | Segment V1 | Segment V2 | Segment V3 | Segment V4 | Basilar artery |
Vps, m/s | control | 0,38 ± 0,05 | 0,39 ± 0,05 | 0,43 ± 0,05 | 0,78 ± 0,11 | 0,97 ± 0,24 |
subgroup а | 0,28 ± 0,17** | 0,69 ± 0,21* | 0,53 ± 0,19 | 0,79 ± 0,22 | 1,08 ± 0,33 | |
Ved, m/s | control | 0,12 ± 0,06 | 0,14 ± 0,04 | 0,18 ± 0,05 | 0,34 ± 0,07 | 0,47 ± 0,10 |
subgroup а | 0,10 ± 0,05 | 0,13 ± 0,04 | 0,20 ± 0,07 | 0,37 ± 0,14 | 0,51 ± 0,16 | |
RI | control | 0,68 ± 0,09 | 0,66 ± 0,08 | 0,63 ± 0,06 | 0,50 ± 0,06 | 0,50 ± 0,04 |
subgroup а | 0,67 ± 0,08 | 0,65 ± 0,06 | 0,65 ± 0,07 | 0,53 ± 0,06 | 0,52 ± 0,06 |
Note: *p < 0.05; **p < 0.003 (the degree of significance is shown compared to the control group).
During the X-ray examination of subgroup а, the flattening of the cervical spine was detected predominantly. The examination results revealed that in subgroup a pediatric patients with S- and C-shaped VA deformities in segment V1 (subgroup a), the said segment was not adjacent to the bone structures of the corresponding lower cervical spine (the vertebral level C6–C7), indicating a congenital genesis of the VA deformities (Fig. 1 a, b).

Fig. 1. Patient K., 12 years old. Ultrasonogram of the congenital S-shaped deformity of segment V1 of the vertebral artery (a) and X-ray images of the cervical spine in the anteroposterior and lateral views (b). The cervical lordosis is flattened; Kimmerle anomaly
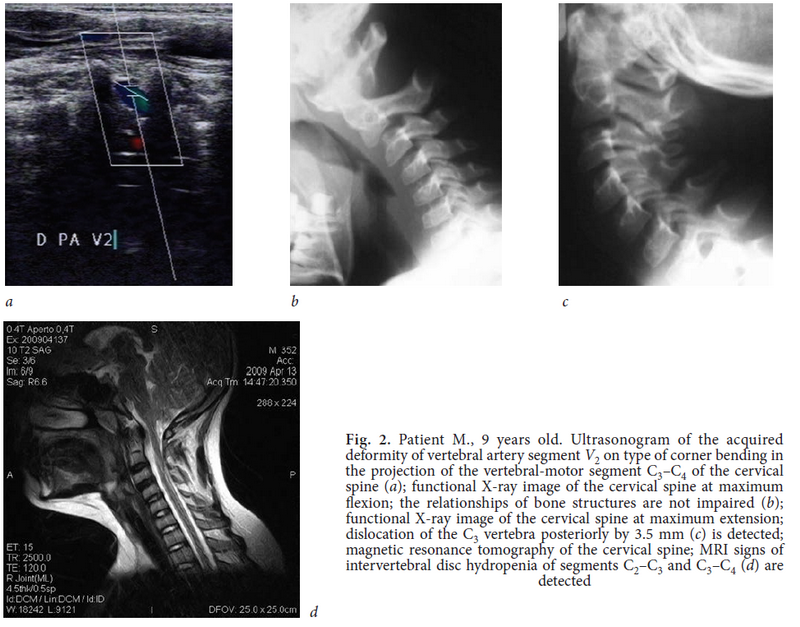
Segment V2 is localized in the bony canal formed by the neural spines of C2–C6. In the subgroup b patients with VA deformities in the form of C-tortuosity and an angular bend in segment V2, ultrasound examination revealed an increase in Vps in this segment to 0.69 ± 0.10 m/s (p < 0.01), whereas blood flow velocity in the other segments of VA and BA did not differ from the control group (Table 3). X-ray examination at the VA deformity level showed instability of segments С2–С3 and С3–С4 of the cervical spine predominantly. During the MRI examination, signs of intervertebral disc hydropenia of the unstable segments was revealed. The results of X-ray images and MRI of the cervical spine confirmed extravasal VA compression due to the influence of bone structures (Fig. 2a, b, c, d).
Table 3. Blood flow velocity of the vertebral arteries and basilar artery in children with C-shaped deformity and angular bend of segment V2 of the vertebral artery (n = 68; М ± m)
Blood flow velocity | Groups | Segment V1 | Segment V2 | Segment V3 | Segment V4 | Basilar artery |
Vps, m/s | control | 0,38 ± 0,05 | 0,39 ± 0,05 | 0,43 ± 0,05 | 0,78 ± 0,11 | 0,97 ± 0,24 |
subgroup b | 0,38 ± 0,07 | 0,69 ± 0,10* | 0,53 ± 0,19 | 0,79 ± 0,22 | 1,08 ± 0,33 | |
Ved, m/s | control | 0,12 ± 0,06 | 0,14 ± 0,04 | 0,18 ± 0,05 | 0,34 ± 0,07 | 0,47 ± 0,10 |
subgroup b | 0,11 ± 0,05 | 0,15 ± 0,04 | 0,20 ± 0,07 | 0,37 ± 0,14 | 0,51 ± 0,16 | |
RI | control | 0,68 ± 0,09 | 0,66 ± 0,08 | 0,63 ± 0,06 | 0,50 ± 0,06 | 0,50 ± 0,04 |
subgroup b | 0,66 ± 0,08 | 0,67 ± 0,06 | 0,65 ± 0,07 | 0,53 ± 0,06 | 0,52 ± 0,06 |
Note: *p < 0.01 (the degree of significance is shown compared to the control group).
Тable 4. Blood flow velocity of the vertebral arteries and basilar artery in children with excessive tortuosity of segment V3 of the vertebral artery (n = 11; М ± m)
Blood flow velocity | Groups | Segment V1 | Segment V2 | Segment V3 | Segment V4 | Basilar artery |
Vps, m/s | control | 0,38 ± 0,05 | 0,39 ± 0,05 | 0,43 ± 0,05 | 0,78 ± 0,11 | 0,97 ± 0,24 |
subgroup c | 0,39 ± 0,07 | 0,51 ± 0,12 | 0,72 ± 0,23* | 0,63 ± 0,23 | 1,02 ± 0,31 | |
Ved, m/s | control | 0,12 ± 0,06 | 0,14 ± 0,04 | 0,18 ± 0,05 | 0,34 ± 0,07 | 0,47 ± 0,10 |
subgroup c | 0,12 ± 0,05 | 0,15 ± 0,04 | 0,30 ± 0,07* | 0,21 ± 0,04 | 0,53 ± 0,14 | |
RI | control | 0,68 ± 0,09 | 0,66 ± 0,08 | 0,63 ± 0,06 | 0,50 ± 0,06 | 0,50 ± 0,04 |
subgroup c | 0,68 ± 0,08 | 0,69 ± 0,07 | 0,66 ± 0,09* | 0,64 ± 0,09 | 0,55 ± 0,06 |
Note: * p < 0.01 (the degree of significance is shown compared to the control group)
The excessive tortuosity of the V3 segment, enveloping the first cervical vertebra, was diagnosed by duplex study of 11 VAs. With this form of VA deformity, there was an increase of Vps to 0.72 ± 0.23 m/s (p < 0.002) in this segment, an increase of Ved to 0.30 ± 0.07 m/s (p < 0.01), and increase of RI to 0.66 ± 0.09 m/s (p < 0.005) compared to the controls. The rest of the blood flow velocities for segments V1, V2, and V4 of VA and BA did not differ from the control group (Table 4). Loop-type deformities were found in 10 VAs. This tortuosity of VA was accompanied by a significant decrease in Vps of segment V3 to 0.31 ± 0.13 m/s (p < 0.002), a decrease in Ved to 0.10 ± 0.04 m/s (p < 0.03), and an increase in Vps of segment V4 to 1.17 ± 0.33 m/s (p < 0.001). Ved increased to 0.55 ± 0.07 m/s (p < 0.0006); Vps acceleration in the BA increased to 1.45 ± 0.34 m/s (p < 0.002); and Ved increased to 0.70 ± 0.24 m/s (p < 0.0001). Blood flow velocity of the segments V1 and V2 did not differ from the control group (Table 5).
Table 5. Blood flow velocity of the vertebral arteries in children with loop deformity of arterial segment V3, (n = 10, М ± m)
Blood flow velocity | Groups | Segment V1 | Segment V2 | Segment V3 | Segment V4 | Basilar artery |
Vps, m/s | control | 0.38 ± 0.05 | 0.39 ± 0.05 | 0.43 ± 0.05 | 0.78 ± 0.11 | 0.97 ± 0.24 |
subgroup c | 0.31 ± 0.06 | 0.48 ± 0.12 | 0.31 ± 0.13* | 1.17 ± 0.33* | 1.45 ± 0.34* | |
Ved, m/s | control | 0.12 ± 0.06 | 0.14 ± 0.04 | 0.18 ± 0.05 | 0.34 ± 0.07 | 0.47 ± 0.10 |
subgroup c | 0.13 ± 0.04 | 0.14 ± 0.05 | 0.10 ± 0.04* | 0.55 ± 0.06* | 0.53 ± 0.14 | |
RI | control | 0.68 ± 0.09 | 0.66 ± 0.08 | 0.63 ± 0.06 | 0.50 ± 0.06 | 0.50 ± 0.04 |
subgroup c | 0.65 ± 0.07 | 0.67 ± 0.07 | 0.51 ± 0.07 | 0.65 ± 0.09 | 0.55 ± 0.06 |
During the X-ray examination, the signs of rotary subluxation of the atlas were revealed in all patients of the subgroup b with excessive VA tortuosity; in two patients, they were seen in combination with Kimmerle anomaly. The data obtained showed extravasal VA compression, leading to malperfusion of the vertebral basilar basin (Fig. 3a, b, c).

Fig. 3. Patient B., 12 years old. Ultrasonogram of the acquired kinking deformity of the V3 segment of vertebral artery (a); X-ray images of the cervical spine through the open mouth revealed asymmetric position of the odontoid process of С2 relative to the lateral mass of the atlas (b); magnetic resonance tomography of the cervical spine showed MRI signs of atlas rotary subluxation (c)
Changes in arterial diameter were found in children with VA lesions of the V1–V4 segments (subgroup d): the diameter on one side had been reduced 2-fold, while on the other side, it had increased 1.5-fold. Peak systolic blood flow velocity was reduced by 51% in the hypoplastic artery and increased by 32% in the dilated contralateral artery.
On X-ray examination, the malformations were revealed (hypoplasia of the C6 vertebral body and synostosis of the articular and spinous processes of C2 and C3 cervical vertebrae). On MRI, hypoplasia of the С4–С5 intervertebral disc and asymmetry of the VA opening diameter were revealed. Thus, the patients had congenital malformations of both soft tissue and bony structures (Fig. 4a, b, c, d).

It should be noted that the Vps changed for all VA deformities, but the nature of these changes over the vessel length had special characteristics. In children with cervical pain syndrome, the values of this index were similar to the control values for the deformity region. With undulating and excessive tortuosity of the artery and angular bend, an increase in Vps is seen in C-, S- and loop-deformity, which indicates a reduction in the value of this index. Above the deformity region, a significant increase in Vps is registered only in patients with C-, S-, and loop-deformity of VA; in other patients, Vps does not differ from the control group.
The results of X-ray examination showed that 87% of cases of cervical pain syndrome in these children were caused by the presence of congenital or acquired VA deformities related to the instability of vertebral-motor segments С2–С3 and С3–С4, congenital abnormalities, including Kimmerle anomaly, synostosis of the articular and spinous processes of C2 and C3, hypoplasia of the C6 vertebral body and intervertebral disc С4–С5, hypertrophy of the transverse processes of the C7, rotary subluxation of the atlas, and impairments of the physiological bend of the cervical spine. MRI of the cervical spine, performed after the radiologically diagnosed instability of segments С2–С3 and С3–С4, and the extravasal compression of VA segment V2, identified by dopplerography, revealed signs of hydropenia of the cervical spine intervertebral discs, usually in children older than 7 years.
Thus, the features underlying the changes in VA blood flow reflect the nature of the arterial lesions and enable assessment of the congenital or acquired genesis of their deformity. Thus, the congenital genesis of segment V1 deformity in the form of C- and S-shaped VA deformities manifests as Vps slowdown in segment V1 and acceleration in segment V3. The congenital VA deformity in the form of a loop-type for segment V3 manifests as a decrease in Vps and Ved in this segment and an increase in Vps and Ved in segment V4 of the VA and BA.
Acquired deformities in the form of an undulating tortuosity and an angular bending in segment V2 were characterized by acceleration of Vps in this segment. The excessive tortuosity of segment V3 was characterized by an increase in Vps, Ved, and RI in this segment.
In the case of congenital VA hypoplasia for segments V1–V4, compensatory expansion of the contralateral homonymous artery occurs.
Conclusions
- A comprehensive study using radiation methods of examination (duplex scanning of VA, X-ray imaging, and MRI if necessary) for the cervical spine in pediatric patients with cervical pain syndrome enabled the determination that 87% of cases were caused by congenital and acquired changes in the bone and soft tissue structures of the cervical spine.
- The results of duplex scanning of the VA in cases of cervical pain syndrome are an indication for special methods of X-ray imaging (functional tests in the position of flexion and extension of the neck and transoral X-ray imaging) to detect instability of the vertebral-motor segments of the cervical spine, congenital anomalies of vertebrae development, rotary subluxation of the atlas, and status of the intervertebral discs.
- The intensity of hemodynamic disorders in the cervical spine of pediatric patients depends on the pathological process of localization in the VA.
- In cases of complete congenital lesion of all the VA segments on one side (hypoplasia), the contralateral artery is always part of the pathological process as that is where the compensatory dilatation develops.
Information on funding and conflict of interest
The authors declare no conflicts of interest related to the manuscript. The study was performed as part of the proactive plan of research and development of the Saratov Research Institute of Traumatology and Orthopedics.
作者简介
Nellya Bakhteeva
Saratov State Medical University n.a. V.I.Razumovsky
编辑信件的主要联系方式.
Email: nelly0812@mail.ru
MD, PhD, professor
Department of Traumatology and Orthopedics 俄罗斯联邦
Tat'yana Ionova
Saratov Scientific and Research Institute of Traumatology and Orthopedics
Email: t.ionova1980@yandex.ru
MD, PhD, doctor of radiation diagnostic dept. 俄罗斯联邦
Valeriy Belonogov
Saratov State Medical University n.a. V.I. Razumovsky
Email: fake@eco-vector.ru
MD, PhD, assistant
Department of Traumatology and Orthopedics 俄罗斯联邦
Sergej Bazhanov
Saratov Scientific and Research Institute of Traumatology and Orthopedics
Email: fake@eco-vector.ru
MD, PhD, senior researcher
the Department of innovative projects in the neurosurgery and spine 俄罗斯联邦
Vladimir Ostrovskij
Saratov Scientific and Research Institute of Traumatology and Orthopedics
Email: fake@eco-vector.ru
MD, PhD, the head of neurosurgery dept. 俄罗斯联邦
参考
- Касаев А.А., Цветкова И.Г., Ялфимов А.Н. Лучевое исследование при нестабильности шейного отдела позвоночника у детей // Проблемы ядерной медицины: материалы 1-го съезда Российского общества ядерной медицины. — Дубна, 1997. — С. 137. [Kasaev AA, Cvetkova IG, Jalfimov AN. Luchevoe issledovanie pri nestabil’nosti shejnogo otdela pozvonochnika u detej. Problemy jadernoj mediciny [conference proceedings]. Dubna; 1997. 137 p. (In Russ.)]
- Бескровная Е.В. Клинико-неврологические и церебральные особенности патологии шейного отдела позвоночника в детском возрасте: Автореф. дис. … канд. мед. наук. — Новосибирск, 2006. [Beskrovnaja EV. Kliniko-nevrologicheskie i cerebral’nye osobennosti patologii shejnogo otdela pozvonochnika v detskom vozraste [dissertation]. Novosibirsk; 2006. (In Russ.)]
- Козел Н.П., Мальчевский В.А. Анализ причин возникновения дистрофических изменений в позвоночно-двигательных сегментах шейного отдела позвоночника у детей, имеющих в анамнезе кривошею // Российский биомедицинский журнал. — 2005. — № 6. — С. 128–129. [Kozel NP, Mal’chevskij VA. Analiz prichin vozniknovenija distroficheskih izmenenij v pozvonochno-dvigatel’nyh segmentah shejnogo otdela pozvonochnika u detej, imejushhih v anamneze krivosheju. Rossijskij biomedicinskij zhurnal. 2005;6:128-129. (In Russ.)]
- Ветрилэ С.Т., Колесов С.В. Краниовертебральная патология. — М.: Медицина, 2007. [Vetrilje ST, Kolesov SV. Kraniovertebral’naja patologija. Moscow: Meditsina; 2007. (In Russ.)]
- Виссарионов С.В., Попов И.В. К вопросу о нестабильности позвоночника: терминологические споры // Травматология и ортопедия России. — 2007. — Т. 44. — № 2. — С. 94–97. [Vissarionov SV, Popov IV. For the question about vertebral instability: terminology disputes. Travmatologija i ortopedija Rossii. 2007; 44(2):94-97. (In Russ.)]
- Кузнецова Л.В., Трапезникова А.М., Скоромец А.П. Клинический полиморфизм остеохондроза позвоночника у детей // TERRA MEDICA: неврология. — 2005. — № 4. — С. 45–47. [Kuznetsova LV, Trapeznikova AM, Skoromec AP. Klinicheskij polimorfizm osteohondroza pozvonochnika u detej. TERRA MEDICA: nevrologija. 2005;(4):45-47. (In Russ.)]
- Fielding JW, Yawkins RJ. Atlanto-axial rotator fixation (fixed rotatory subluxation of the atlanto-axial joint). J Bone & Jt Surg. 1977;59(1):37-44. doi: 10.2106/00004623-197759010-00005.
- Максимов Ю.Н., Хайбуллина Д.Х. Проблема выявляемости вертеброгенной патологии в детском и подростковом возрасте // Вертеброневрология. — 1998. — № 1. — С. 42–44. [Maksimov JuN, Hajbullina DH. Problema vyjavljaemosti vertebrogennoj patologii v detskom i podrostkovom vozraste. Vertebronevrologija. 1998;(1):42-44. (In Russ.)]
- Heggenes MH, Doherty BJ. The Trabecular Anatomy of the axis. Spine. 1993;18:1945-1949. doi: 10.1097/00007632-199310001-00003.
- Митрохин А.Н., Кузина И.Р. Комплексная лучевая диагностика детей, страдающих головными болями, при нестабильности и остеохондрозе шейного отдела позвоночника. Новые горизонты // Невский радиологический форум. — СПб.: ЭЛБИ-СПб, 2007. — С. 621–622. [Mitrohin AN, Kuzina IR. Kompleksnaja luchevaja diagnostika detej, stradajushhih golovnymi boljami, pri nestabil’nosti i osteohondroze shejnogo otdela pozvonochnika. Novye gorizonty. Nevskij radiologicheskij foruma. Saint Petersburg: JeLBI-SPb; 2007:621-622. (In Russ.)]
- Бахтеева Н.Х., Ионова Т.А., Григорьева А.А. Результаты обследования детей с цервикальным болевым синдромом // Травматология и ортопедия России. — 2010. — Т. 55. — № 1. — С. 38–42. [Bahteeva NH, Ionova TA, Grigor’eva AA. Examination results of children with cervical syndrome. Travmatologija i ortopedija Rossii. 2010;55(1):38-42. (In Russ.)]
补充文件







