Функциональный анализ клТ-ДНК в природно-трансформированных растениях, последние результаты и общие соображения
- Авторы: Оттен Л.1
-
Учреждения:
- Институт молекулярной биологии растений
- Выпуск: Том 14, № 4 (2016)
- Страницы: 26-31
- Раздел: Статьи
- Статья получена: 03.02.2017
- Статья опубликована: 15.12.2016
- URL: https://journals.eco-vector.com/ecolgenet/article/view/5986
- DOI: https://doi.org/10.17816/ecogen14426-31
- ID: 5986
Цитировать
Полный текст
Аннотация
Резюме: Мы описали структуру четырех различных клТ-ДНК N. tomentosiformis, являющегося предковой формой N. tabacum, и обнаружили несколько интактных ORF. Среди них TB-mas2’ и TA-rolC были тестированы на функциональность. TB-mas2’ кодирует фермент синтеза дезоксифруктозилглутамина (DFG). Некоторые сорта N. tabacum характеризуются очень высоким уровнем экспрессии TB-mas2’ и образуют DFG в корнях. TA-rolC биологически активен и будучи экспрессирован под контролем сильного конститутивного промотора вызывает изменения ростовых характеристик N. tabacum. Основываясь на данных о структуре и функции клТ-ДНК предожена теоретическая модель происхождения и эволюции природно-трансгенных растений, которая может быть использована при планировании последующих исследований.
Ключевые слова
Полный текст
INTRODUCTION
Pathogenic strains of Agrobacterium induce Crown galls and hairy roots on a large number of plant species. During the infection process, generally taking place wound sites, agrobacteria introduce one or several DNA fragments derived from tumor or root-inducing (Ti or Ri) plasmids. These DNAs are called T-DNAs (transferred DNAs, in case two T-DNAs are transferred these are called TA and TB or TL and TR) and contain several genes that will subsequently be expressed in the plant cells, leading to genetically transformed (transgenic) tissues. Many different Agrobacterium strains have been described and each contains its own type of T-DNA. T-DNAs are not always transferred intact, and many cases are known of partially duplicated tandem structures (either direct or indirect repeats, due to ligation of T-DNAs before insertion into the plant genome). Ti and Ri plasmids evolve most probably by horizontal gene transfer due to plasmid exchange between agrobacteria followed by recombination. Thus, the T-DNAs are mosaic structures and their evolution is difficult to reconstruct [1]. Many types of Ti and Ri plasmids exist and undoubtedly, many remain to be discovered. The T-DNA genes encode two major types of function. The first group encodes enzymes leading to the production of small conjugated molecules, called opines [2]. Opines are found in tumors and hairy roots, often at high concentrations, and many different types have been identified. Different substrates can be used for their production, all are important metabolites like amino acids, sugars and α-keto acids. Opines are not found in normal plants. They are secreted from the transgenic tissues and taken up by the agrobacteria. Genes coding for opine uptake and opine degradation are located on the Ti/Ri plasmids and allow the bacterium to recover the original molecules. The second group of T-DNA genes enhances opine production by stimulating division and growth of the transformed cells. Among the genes of the Ti plasmids, the iaaM and iaaH genes code for auxin synthesis, the ipt gene for cytokinin synthesis, together they lead to undifferentiated growth. In the case of Ri plasmids, the rolA, rolB and rolC genes induce hairy roots by an as yet unknown mechanism [3, 4]. The Agrobacterium Ti/Ri-based transformation system provides an important selective advantage for agrobacteria, since other bacteria normally cannot use opines. The genetic manipulation of plants by pathogenic Agrobacterium strains aimed at providing important metabolites to the bacterium has been called genetic colonization [5].
Early in Agrobacterium research it was noted that several species of the Nicotiana genus contained T-DNA sequences [6, 7]. One of these, N. glauca (tree tobacco), was studied in more detail and found to contain an imperfect inverted repeat (called cellular T-DNA or cT-DNA) that resembled part of an A. rhizogenes T-DNA. This meant that somewhere in the evolutionary history of this species an Agrobacterium transformation event had occurred. Most likely, hairy roots were induced on a N. glauca ancestor, and spontaneously regenerated into fertile plants. The full structure of the N. glauca cT-DNA was only established much later. It consists of a partial inverted repeat, with an intact opine synthesis gene, the mikimopine synthase (mis) gene. Although this gene was shown to encode an active enzyme as demonstrated by expression in E. coli, no mikimopine could be found in N. glauca plants [8]. An extensive search in other plant species led to the discovery of an imperfect direct cT-DNA repeat in Linaria vulgaris [9, 10]. Later, complete cT-DNAs were also described for N. tabacum (tobacco), N. tomentosiformis (the paternal ancestor of N. tabacum), N. tomentosa and N. kawakamii. Full sequencing of the N. tomentosiformis genome revealed the presence of no less than 4 different cT-DNAs (TA to TD). All are imperfect inverted repeats. These cT-DNAs were probably introduced by four successive transformations of an ancestor Nicotiana species by four different A. rhizogenes strains. The four T-DNAs partly resemble T-DNAs of known A. rhizogenes strains like 1724, but they also show several new features [11]. Interestingly, the TC cT-DNA was lost in N. tabacum by deletion, and the TA cT-DNA underwent a partial deletion in some N. tabacum cultivars, showing that cT-DNAs can undergo both small and large evolutionary changes. More recently, cT-DNAs were found in the agronomically important species Ipomoea batatas (sweet potato) [12]. It is likely that all cT-DNAs are derived from A. rhizogenes infections, with initial induction of hairy roots. Relatively little is known about the mechanism of induction and growth of hairy roots [13]. The Nicotiana, Linaria and Ipomoea groups thus contain natural GMOs, and other such plants will likely be discovered in the future.
One important question concerning these natural GMOs is whether the cT-DNAs are expressed, whether they might influence growth and possibly provide a selective advantage. In this paper I would like to discuss the evidence obtained so far, and provide a theoretical model for the origin and further evolution of natural GMOs.
Many open reading frames have been found on natural cT-DNAs. Some of these could be important in the growth or metabolism of natural GMOs. The possible role of cT-DNA genes in the origin and growth of plants has been studied by different groups. I first will discuss opine synthesis genes, then other cT-DNA genes.
Activity of cT-DNA opine synthesis genes
As mentioned above, the N. glauca cT-DNA mis gene encodes an active enzyme, but no mikimopine was found in N. glauca plants. In N. tomentosiformis, the TC cT-DNA contains an intact octopine synthase-like (ocl) gene. This gene was placed under strong, constitutitive promoter control (yielding 2 × 35S-ocl) and transiently expressed in N. benthamiana. Since a priori the opine structure could not be predicted, a negative staining method was used which can reveal the presence of opines when they are produced at high levels. A control gene, vitopine synthase (2 × 35S-vis), was tested in the same way and gave a positive response. Therefore, the ocl gene may not encode an active enzyme [11]. A third intact opine synthesis gene on the TB cT-DNA of N. tomentosiformis and N. tabacum is the mannopine synthase 2’ (TB-mas2’) gene. This gene was already known from T-DNAs of some A. tumefaciens and A. rhizogenes strains and encodes the synthesis of desoxyfructosylglutamine (DFG) in tumors and hairy roots. Notable differences exist between the A. tumefaciens and A. rhizogenes mas2’ genes, the TB-mas2’ gene belongs to the A. rhizogenes type. Using the same approach as for the ocl gene, it was found that TB-mas2’ expression in N. benthamiana leads to DFG synthesis [11]. The next step was to look for DFG in Nicotiana. The expression levels of the TB-mas2’ genes of N. tomentosiformis and most cultivars of N. tabacum were extremely low, but surprisingly, some tobacco cultivars showed very high levels of expression. The promoters of all TB-mas2’ genes being identical, the differences in expression might be due to other genes (possibly transcription factor genes) present in high expression cultivars. Crosses between high and low expressors showed that the high expression phenotype behaved like a single mendelian factor. The expression pattern of the TB-mas2’ gene was studied by the gene reporter approach (using a TB-mas2’ promoter-GUS construct and a control A4-mas2’ promoter-GUS construct expressed in N. benthamiana). These studies showed that the A4-mas2’ and TB-mas2’ genes are both preferentially expressed in root tips (in the case of A4-mas2’ most likely in the tips of the hairy roots). This suggested that root tips of high expression tobacco cultivars might contain DFG, and this was indeed shown to be the case. DFG is an Amadori compound and is highly unstable because of rearrangements, but the DFG product in tobacco roots could unambiguously be identified by mass spectroscopy [14]. This was the first demonstration of opine production in a natural transgenic plant and showed that when looking for opines in natural GMOs, it might be helpful to first identify sites of high opine gene expression using reporter genes, and then search for the expected opines at these sites. Similar studies might be carried out with opine genes of other natural GMOs.
Study of cT-DNA genes of the plast type
Among the other intact cT-DNA genes, only a few have been investigated so far. The first to be studied were torf13 and trolC from the TA region of tobacco (these genes can also be called TA-orf13 and TA-rolC but the older names will be used here to avoid confusion). Both belong to a large family of distantly related genes, the plast genes (for phenotypic plasticity) [15] and are found on the T-DNAs of A. rhizogenes. The rolC gene is directly involved in hairy root induction, whereas the torf13 gene is not essential but can stimulate hairy root induction. The torf13 was shown to have biological activity in root induction assays on tobacco leaf disks [16] and on carrot disks [17]. However, neither the transfer of torf13 from tobacco cultivar Wisconsin to cultivar Samsun nn which has a partial TA deletion and lacks torf13 genes, nor its stable expression under 2 × 35S promoter control in tobacco showed any morphological effects of this gene (Otten, unpublished results).
The trolC gene was tested by stable expression in tobacco under control of a dexamethasone-(DEX) inducible promoter in tobacco (dex-trolC). As a control, the A4-rolC gene from A. rhizogenes was used (dex-A4-rolC). The induction of both genes led to similar phenotypes: reduction of seedling growth, mosaic leaves with light and dark green areas, and accumulation of starch in leaves. Roots lost their root hairs and isolated roots became capable of growing on medium with low concentrations of sucrose. Isolated dex-trolC roots eventually stopped their growth whereas dex-A4-rolC roots continued to grow [18]. Thus trolC has biological activity.
Some theoretical considerations concerning natural GMOs
The studies mentioned above show that at least some cT-DNA genes are biologically active. This raises the question about their possible function in the emergence and subsequent development of natural GMO plants. Although it is possible that natural GMOs are chance products of evolution and do not differ from their non-transgenic ancestors, this seems unlikely because the regenerants contain T-DNA genes like rolA, rolB and rolC known to influence plant development. The problem of cT-DNA gene function clearly needs to be separated in two parts: their role in the initial regenerants, and during the further evolution of such plants.
Origin of natural GMOs
Natural GMOs result from the regeneration of tissues containing A. rhizogenes-type T-DNAs. Nothing is known about the regeneration of hairy roots to fertile plants under natural conditions, nor about the frequency of occurrence. Regeneration requires two conditions: the plant must be able to produce shoots from hairy roots, and the T-DNA structure must allow and/or favor such regeneration. Some species are well known for their capability to regenerate shoots from roots. Interestingly, Linaria is one of these. Tobacco can also regenerate easily: it was initially selected for tissue culture because of its excellent regeneration properties. Additional natural GMOs might be found by deep sequencing analysis of plants with a high regeneration capacity. It is likely that efficient shoot regeneration from hairy roots requires one or more special combinations of T-DNA genes in the hairy root tissues, each at specific expression levels. In this context it is interesting to note that the expression “hairy root” is extremely ill-defined in terms of root morphology and growth properties, and may cover different types of modified roots from highly abnormal to nearly normal. In order to test the hypothesis that some T-DNAs could lead to spontaneous shoot regeneration of hairy roots, one might choose as an example the TA region found in some Nicotiana species. One then needs to carry out the following experiments: 1. Identify a Nicotiana species close to the ancestor that received the T-DNA to be tested. In the case of the TA cT-DNA this could be N. tomentosa, which lacks the TA region. 2. Reconstruct the structure of the T-DNA that was introduced in the ancestor. The present TA sequences are partially mutated, degenerated and diverged (divergence between the repeats is 1.2 %). Reconstruction of the original TA T-DNA should be possible by comparison of the sequences of the repeated areas, eventually by sequence analysis of TAs from other Nicotiana species or accessions. 3. Infect the putative ancestor (here: N. tomentosa) with an A. rhizogenes strain carrying the reconstructed TA T-DNA. Since the TA region carries rolA, rolB and rolC genes, which are known to be essential and sufficient for hairy root induction, it can be expected that hairy roots will form. In the case of TA, these hairy roots will produce mikimopine. Each hairy root constitutes a clone, and many clones with different T-DNA structures and expression patterns might be generated at the infection site. It should be noted that we do not really know how much genetic variation can be expected for these clones, and whether initiation and growth of hairy roots from infected areas is subject to strong selection for certain T-DNA gene combinations or not. 4. Find conditions under which some of the hairy root clones form shoots. An extensive study on the phenotypes of these shoots, their cT-DNA structures and expression levels can provide us with a theory concerning the conditions (cT-DNA structure and expression) that will allow spontaneous regeneration of hairy roots and the probabilities of such conditions. 5. Study the fertility of a range of spontaneous regenerants and their capacity to generate progeny with the putative non-transformed ancestor. If some lines are fertile, but reproductively isolated from the ancestor, they are by definition new species, and the transformed plants might survive as natural GMOs. Reproductive isolation could occur by changes in flower structure or flowering time. It is also possible that the initial regenerants mainly reproduce by selfing (as in the case of tobacco), this would rapidly lead to clonal populations of transformed plants. The species within the Tomentosa group of the genus Nicotiana might potentially be explained by successive T-DNA transfer events. It is quite possible that horizontal gene transfer by Agrobacterium can create new species, which would enlarge the famous “origin of species by natural selection” [19] with an “origin of species by horizontal gene transfer”. On the other hand, if the initial GMOs still formed fertile progeny with their ancestors, they would have disappeared from natural populations, unless the cT-DNA genes provided a selective advantage. After discussing the emergence of the initial natural GMO plants, their possible selection and maintenance over longer periods should now be considered.
Maintenance of natural GMOs
It can be assumed that the genes of the T-DNAs that gave rise to natural GMOs were intact and led to classical hairy roots on susceptible plant species. However, present-day cT-DNAs carry various types of deleterious mutations. The TA region of N. tomentosiformis is strongly modified: only torf13 and trolC are intact. The mutations could result from infection by an A. rhizogenes strain with an incomplete and mutated TA T-DNA, or from mutations that occurred in a hairy root and then allowed shoot formation. A third possibility is that the initial plants carried an intact TA cT-DNA and were relatively abnormal, with later mutations improving growth. If the original regenerant was reproductively isolated from the ancestor, no particular selective advantage was required for initial multiplication of such plants. However, subsequent cT-DNA mutations might progressively produce more efficient plants. Interestingly, the TA region is partially deleted in some tobacco cultivars, with loss of the two torf13 gene copies but leaving the trolC genes intact. This could be fortuitous (gene erosion) or result from selection against some detrimental torf13 effects.
Are natural GMOs a part of the Agrobacterium genetic colonization strategy?
Tumors and hairy roots are opine factories and serve as cheap sources of sugars, amino acids and α-keto acids for the inciting Agrobacterium strains. Hairy root regenerants could continue to secrete opines from their roots which would lead to a large overall increase in opine production. If in addition, such isolated plants would be able to reproduce (either vegetatively as in the case of Linaria or sexually as in the case of Nicotiana) they would form regularly reproducing populations, thereby creating an even greater source of opines. Transgenic plants with active opine synthesis (most probably in the roots) would constitute a rich and stable source of opines for agrobacteria and might be considered as the ultimate step in genetic colonization. However, if opine production would diminish plant growth, these opine-producing plants would undergo selection for loss of opine synthesis and the benefit would only be temporary. On the other hand, a relatively low amount of opine production would not be detrimental to the plant but still of advantage for the bacterium. These considerations also raise the question of a possible advantage of opine production for the plant, for example by favoring the growth of plant-beneficial bacteria. For the moment, this seems to be a highly speculative possibility.
With regard to opine synthesis, one may expect two steps in the appearance of natural GMOs: 1. Regeneration of intact plants from hairy roots producing relatively large amounts of opines. 2. Appearance of mutants with less or no opine synthesis. In order to test the influence of opine synthesis on plant growth, opine synthesis genes of natural GMOs might be restored by CRISPR-Cas9 (in the case of the TA region, this would involve restoration of the two mis gene copies), followed by fitness comparisons with the non-restored plants. Any hypothesis proposing an initial selective advantage of natural opine-producing GMOs for Agrobacterium would also require a study of Agrobacterium populations in the rhizosphere of natural GMOs that secrete opines. So far, opine production has only been found in a few tobacco cultivars (like Russian Burley), these express the TB-mas2’ gene to very high levels with concomitant production of DFG [14]. More studies are required on the regulation of the TB-mas2’ gene, and the amounts of DFG that are synthesized and secreted, both under artificial and natural conditions. DFG can also be used by other bacteria and therefore does not constitute an ideal case [20]. It appears that the production of DFG in the high expression cultivars is a secondary phenomenon, since the TB-mas2’ genes of the tobacco ancestor N. tomentosiformis and most other tobacco cultivars show only very low expression. High TB-mas2’ expression might have been selected by tobacco growers and not occur in nature. Further studies will be required to determine whether DFG-producing tobacco plants occur in nature, whether they actively secrete DFG and whether this favors growth of agrobacteria or other microorganisms.
The possibility that agrobacteria use natural GMOs as opine sources could also imply a role for plast genes. In the case of trolC, it has been shown that this gene favors sucrose uptake [18]. As glucose and fructose are precursors for DFG, such plast genes might improve DFG production, especially if they are expressed in roots like the TB-mas2’ gene. It is therefore important to learn more about the tissue-specific expression of the trolC gene, and its possible effect on DFG production. CRISPR-Cas9 may be used to remove the trolC genes or the TB-mas2’ gene from the DFG-producing tobacco cultivars.
Conclusion
The various possible steps in the evolution of natural GMOs are summarized in Figure
- Hopefully, some of the hypotheses evoked in this paper can be tested. The results will undoubtedly tell us more about the origin and the further evolution of the natural GMOs.
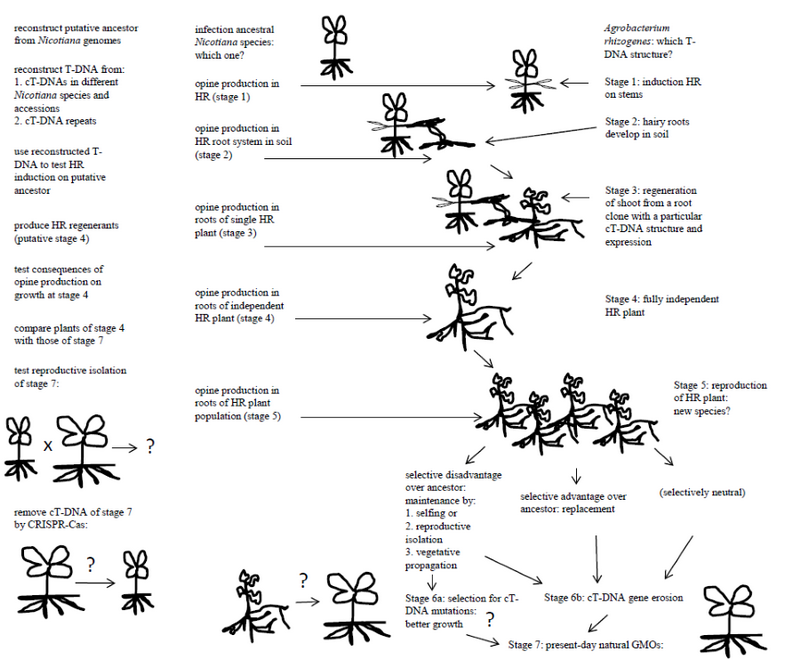
Fig. 1. Different hypothetical stages in the origin and further evolution of natural GMOs. On the left: possible ways to study the hypothetical events. The plant forms are symbolical and intend to show changes in morphology and physiology at the different steps. HR: hairy root
Рис. 1. Различные гипотетические этапы возникновения и дальнейшей эволюции природных ГМО. Слева: возможные пути для изучения этих гипотетических событий. Формы растений являются символическими и показывают изменения в морфологии и физиологии на различных этапах. HR: косматые корни
Об авторах
Леон Оттен
Институт молекулярной биологии растений
Автор, ответственный за переписку.
Email: leon.otten@ibmp-cnrs.unistra.fr
Россия
Список литературы
- Otten L, De Ruffray R. Agrobacterium vitis nopaline Ti plasmid pTiAB4: relationship to other Ti plasmids and T-DNA structure. Mol Gen Genet. 1994;245:493-505. doi: 10.1007/BF00302262.
- Vladimirov IA, Matveeva TV, Lutova LA. Opine biosynthesis and catabolism genes of Agrobacterium tumefaciens and Agrobacterium rhizogenes. Russ J Genet. 2015;51:121-129. doi: 10.1134/S1022795415020167.
- Spena A, Schmülling T, Koncz C, Schell JS. Independent and synergistic activity of rol A, B and C loci in stimulating abnormal growth in plants. EMBO J. 1987;6:3891-3899.
- Nilsson O, Olsson O. Getting to the root: the role of the Agrobacterium rhizogenes rol genes in the formation of hairy roots. Physiol Plant. 1997;100, 463-473. doi: 10.1111/j.1399-3054.1997.tb03050.x.
- Schell J, Van Montagu M, De Beuckeleer M, et al. Interactions and DNA Transfer between Agrobacterium tumefaciens, the Ti-Plasmid and the Plant Host. P Roy Soc Lond B Bio. 1979;204:251-266. doi: 10.1098/rspb.1979.0026.
- White F, Garfinkel D, Huffman GA, et al. Sequences homologous to Agrobacterium rhizogenes T-DNA in the genomes of uninfected plants. Nature. 1983;301:348-350. doi: 10.1038/301348a0.
- Furner IJ, Huffman GA, Amasino RM, et al. An Agrobacterium transformation in the evolution of the genus Nicotiana Nature. doi: 10.1038/319422a0.
- Suzuki K, Yamashita I, Tanaka N. Tobacco plants were transformed by Agrobacterium rhizogenes infection during their evolution. Plant J. 2002;32:775-787. doi: 10.1046/j.1365-313X.2002.01468.x.
- Matveeva TV, Bogomaz DI, Pavlova OA, et al. Horizontal gene transfer from genus Agrobacterium to the plant Linaria in nature. MPMI. doi: 10.1094/MPMI-07-12-0169-R.
- Matveeva TV, Lutova LA. Horizontal gene transfer from Agrobacterium to plants. Front Plant Sci. 2014;11(5):326. doi: 10.3389/fpls.2014.00326.
- Chen K, Dorlhac de Borne F, Szegedi E, Otten L. Deep sequencing of the ancestral tobacco species Nicotiana tomentosiformis reveals multiple T-DNA inserts and a complex evolutionary history of natural transformation in the genus. Nicotiana Plant J. 2014;80:669-682. doi: 10.1111/tpj.12661.
- Kyndt T, Quispe D, Zhai H, et al. The genome of cultivated sweet potato contains Agrobacterium T-DNAs with expressed genes: an example of a naturally transgenic food crop. Proc Nat Acad Sci USA. 2015;112:5844-5849. doi: 10.1073/pnas.1419685112.
- Meyer A, Tempé J, Costantino P. Hairy root: a molecular overview. Functional analysis of Agrobacterium rhizogenes T-DNA genes. In Plant-Microbe Interactions, Vol. 5 (G. Stace, Keen N.T., eds). St. Paul, Minnesota: APS Press; 2000:93-139.
- Chen K, Dorlhac de Borne F, Julio E, et al. Root-specific expression of opine genes and opine accumulation in some cultivars of the naturally occurring GMO Nicotiana tabacum. Plant J. 2016; in press.
- Levesque H, Delepelaire P, Rouzé P, et al. Common evolutionary origin of the central portion of the Ri TL-DNA of Agrobacterium rhizogenes and the Ti T-DNAs of Agrobacterium tumefaciens. Plant Mol Biol. 1988;11:731-744. doi: 10.1007/BF00019514.
- Aoki S, Syono K. Function of Ngrol genes in the evolution of Nicotiana glauca conservation of the function of NgORF13 and NgORF14 after ancient infection by an Agrobacterium rhizogenes-like ancestor. Plant Cell Physiol. 1999;40:222-230. doi: 10.1093/oxfordjournals.pcp.a029531.
- Fründt C, Meyer AD, Ichikawa T, Meins F. A tobacco homologue of the Ri-plasmid orf13 gene causes cell proliferation in carrot root disks. Mol Gen Genet. 1998;259:559-68. doi: 10.1007/s004380050849.
- Mohajjel-Shoja H, Clément B, Perot J, et al. Biological activity of the Agrobacterium rhizogenes-derived rolC gene of Nicotiana tabacum and its functional relationship to other plast genes. MPMI. 2011;24:44-53. doi: 10.1094/MPMI-06-10-0139.
- Darwin C. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life, London, John Murray. 1859.
- Baek CH, Farrand SK, Park DK, et al. Genes for utilization of deoxyfructosyl glutamine (DFG), an amadori compound, are widely dispersed in the family Rhizobiaceae. FEMS Microbiol Ecol. 2005;53:221-233. doi: 10.1016/j.femsec.2004.12.008.
Дополнительные файлы












